23valent Pneumococcal Polysaccharide Vaccine Market Report
Published Date: 31 January 2026 | Report Code: 23valent-pneumococcal-polysaccharide-vaccine
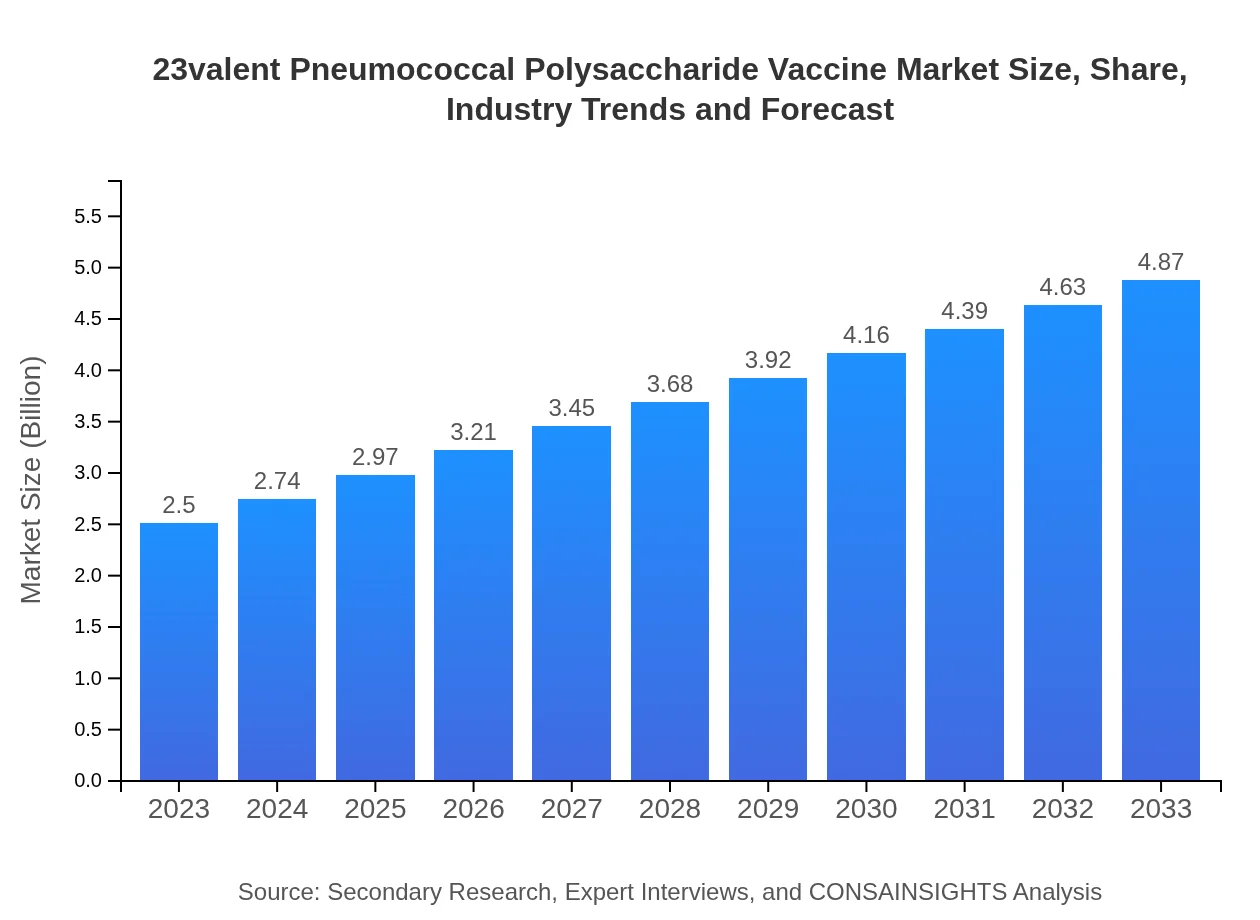
23valent Pneumococcal Polysaccharide Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the 23valent Pneumococcal Polysaccharide Vaccine market, encompassing key insights, trends, and forecasts from 2023 to 2033, aimed at understanding its growth dynamics and market potential.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $4.87 Billion |
| Top Companies | Pfizer Inc., Merck & Co., Inc., Sanofi Pasteur, GlaxoSmithKline plc |
| Last Modified Date | 31 January 2026 |
23valent Pneumococcal Polysaccharide Vaccine Market Overview
Customize 23valent Pneumococcal Polysaccharide Vaccine Market Report market research report
- ✔ Get in-depth analysis of 23valent Pneumococcal Polysaccharide Vaccine market size, growth, and forecasts.
- ✔ Understand 23valent Pneumococcal Polysaccharide Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in 23valent Pneumococcal Polysaccharide Vaccine
What is the Market Size & CAGR of 23valent Pneumococcal Polysaccharide Vaccine market in 2023?
23valent Pneumococcal Polysaccharide Vaccine Industry Analysis
23valent Pneumococcal Polysaccharide Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
23valent Pneumococcal Polysaccharide Vaccine Market Analysis Report by Region
Europe 23valent Pneumococcal Polysaccharide Vaccine Market Report:
The European market is expected to grow from USD 0.68 billion in 2023 to USD 1.33 billion by 2033. Stringent health policies, coupled with proactive immunization strategies, position Europe as a leader in the adoption of the PPSV23 vaccine.Asia Pacific 23valent Pneumococcal Polysaccharide Vaccine Market Report:
The Asia Pacific region represents a significant market for 23valent Pneumococcal Polysaccharide Vaccine with a market value projected at USD 0.50 billion in 2023, expecting growth to USD 0.97 billion by 2033. This growth is primarily due to the increasing population and focus on vaccination campaigns to combat pneumococcal diseases.North America 23valent Pneumococcal Polysaccharide Vaccine Market Report:
North America shows a robust PPSV23 market with a current value of USD 0.90 billion in 2023 and expected to grow to USD 1.76 billion by 2033. Strong healthcare systems and government-funded immunization programs are supporting this growth, particularly among the elderly population.South America 23valent Pneumococcal Polysaccharide Vaccine Market Report:
In South America, the market is currently valued at approximately USD 0.21 billion in 2023, anticipated to reach USD 0.41 billion by 2033. The growth in this region is driven by enhancing healthcare infrastructures and rising health awareness regarding preventive measures against infectious diseases.Middle East & Africa 23valent Pneumococcal Polysaccharide Vaccine Market Report:
The market in the Middle East and Africa stands at USD 0.21 billion in 2023, projected to increase to USD 0.41 billion by 2033. Initiatives to bolster healthcare access and combat infectious diseases are driving market growth in this region.Tell us your focus area and get a customized research report.
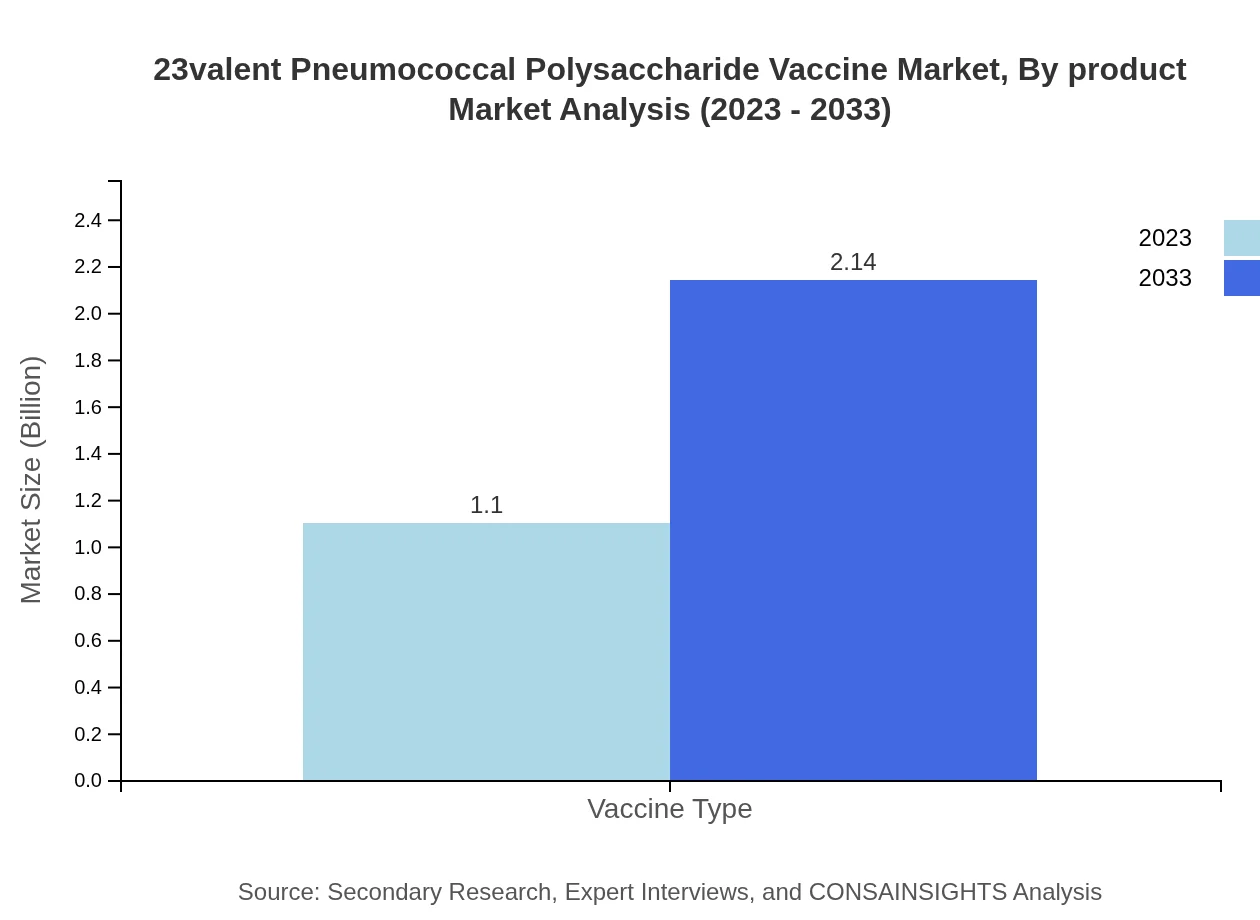
23valent Pneumococcal Polysaccharide Vaccine Market Analysis By Product
The vaccine types within the market include the 23-valent polysaccharide vaccine, which is expected to grow from a size of USD 1.10 billion in 2023 to USD 2.14 billion by 2033. This growth reflects the vaccine's importance in public health strategies for preventing pneumococcal diseases.
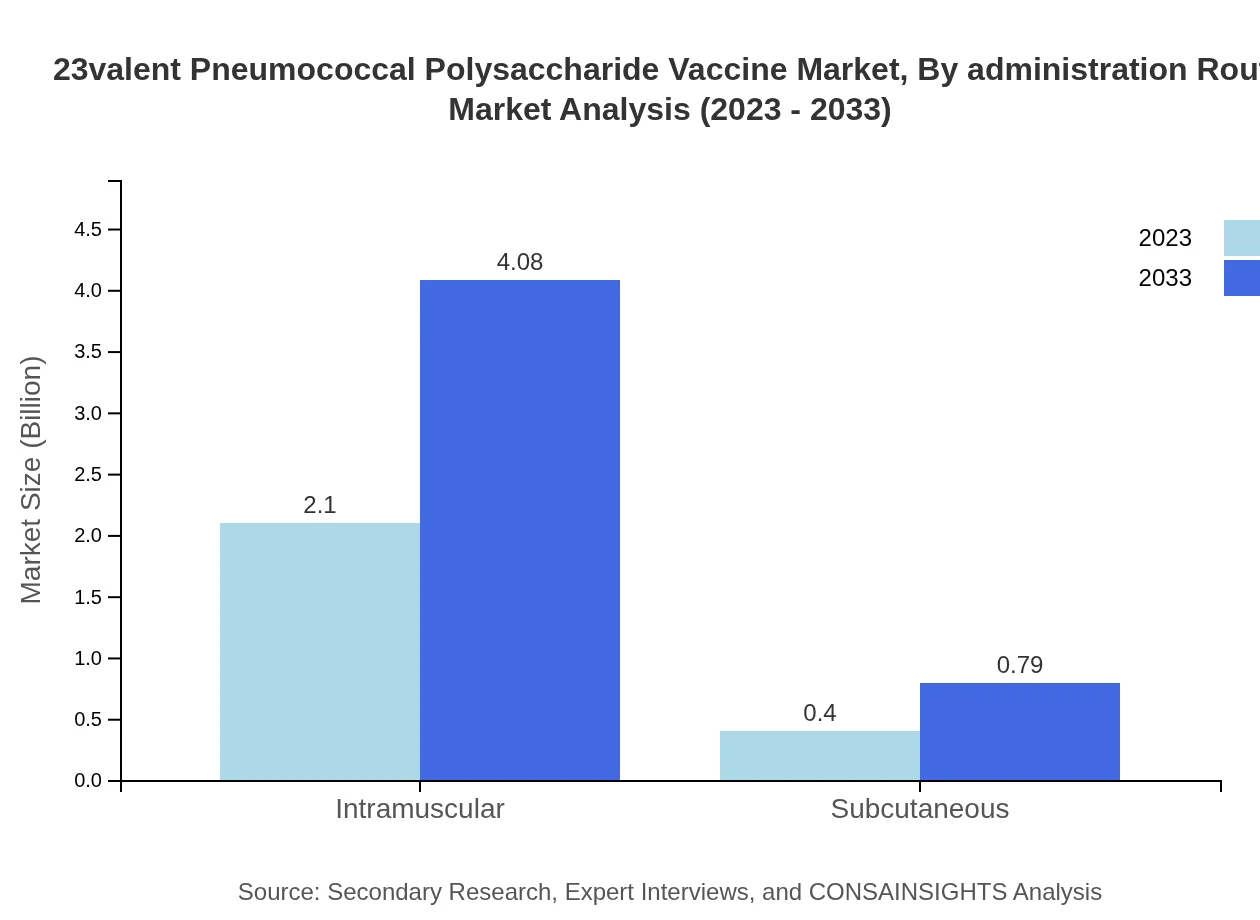
23valent Pneumococcal Polysaccharide Vaccine Market Analysis By Administration Route
Intramuscular administration is the dominant route, comprising 83.83% of the share and valued at USD 2.10 billion in 2023, with projections to reach USD 4.08 billion by 2033. Subcutaneous routes represent a smaller share but are anticipated to grow alongside the increase in vaccine utilization.
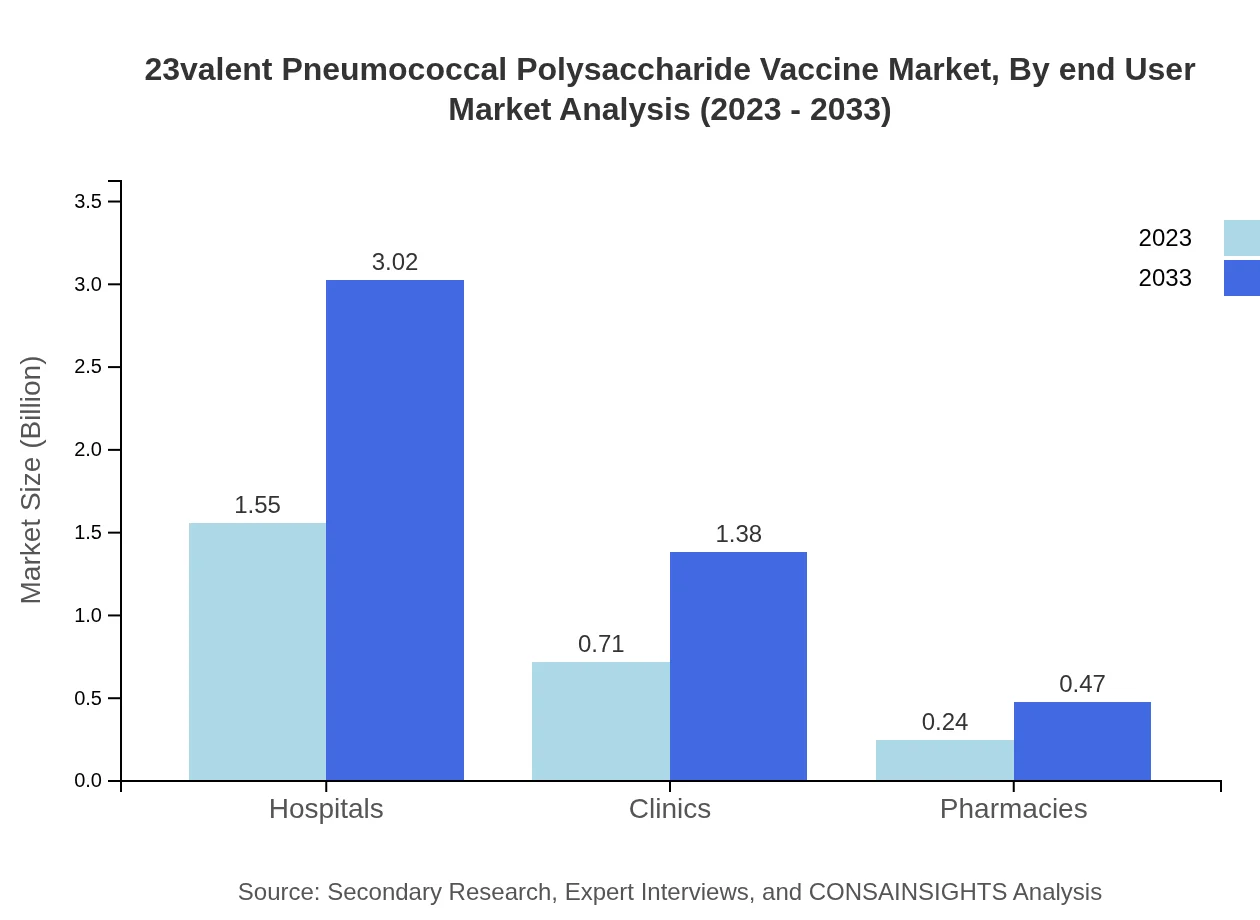
23valent Pneumococcal Polysaccharide Vaccine Market Analysis By End User
Hospitals remain the largest end-user segment, projecting a market size of USD 1.55 billion in 2023 to USD 3.02 billion in 2033, holding 62.07% of the total market share. Clinics and pharmacies are also noteworthy segments, contributing significantly to the overall consumption of the vaccine.
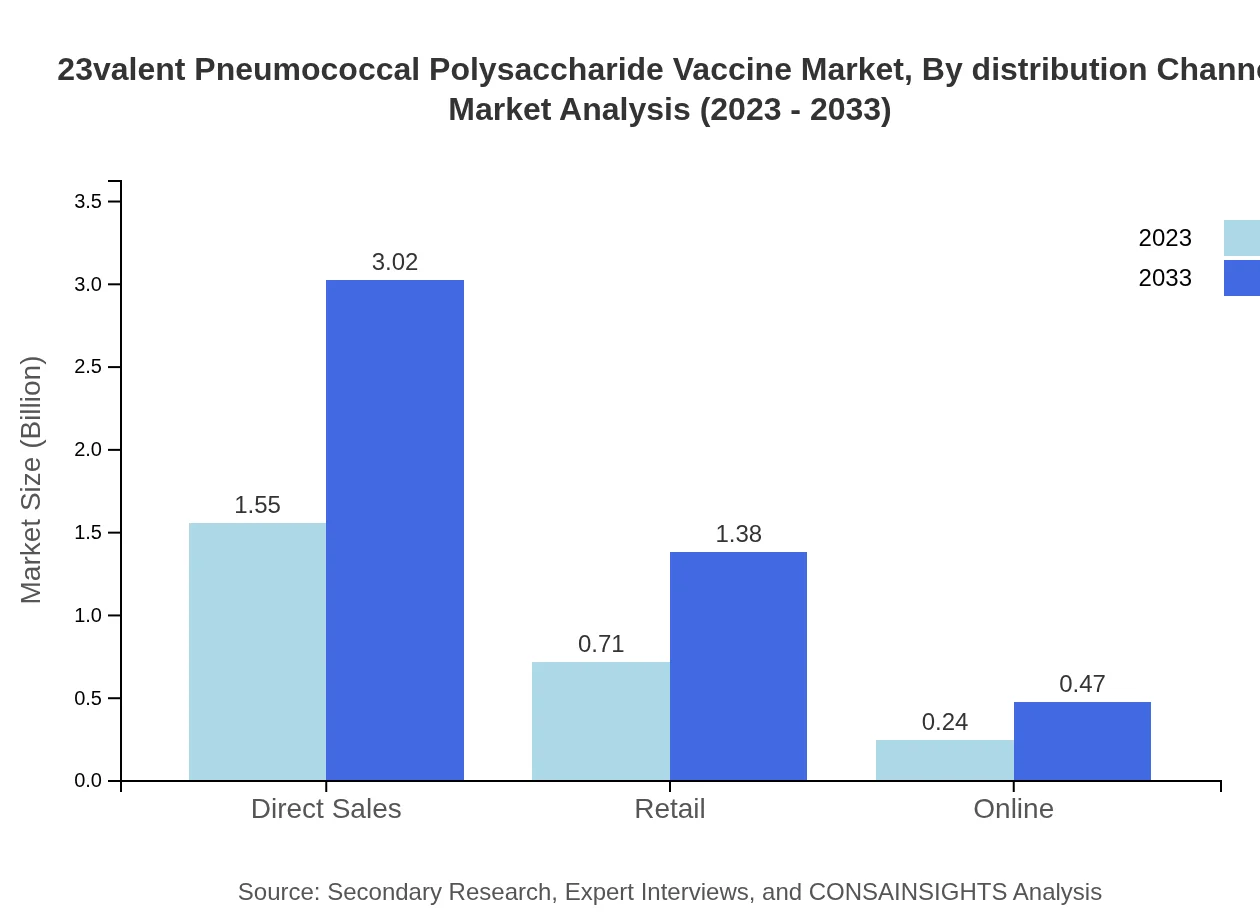
23valent Pneumococcal Polysaccharide Vaccine Market Analysis By Distribution Channel
Direct sales dominate the distribution channels, making up USD 1.55 billion in 2023 and growing to USD 3.02 billion by 2033, reflecting 62.07% of market share. Retail and online sales are also emerging distribution channels, increasing their market footprints accordingly.
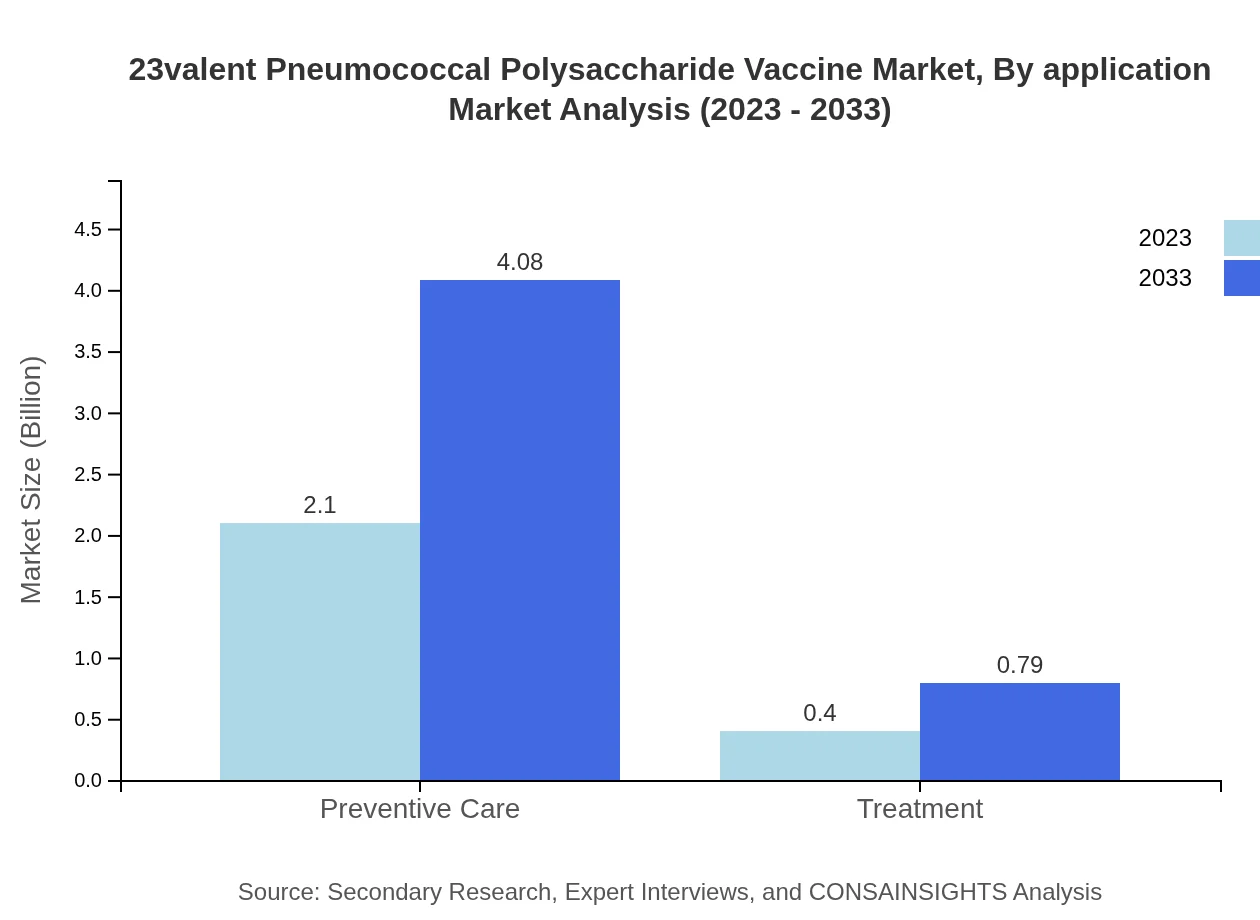
23valent Pneumococcal Polysaccharide Vaccine Market Analysis By Application
The application of the vaccine is primarily in preventive care, composing 83.83% of the market share with a size of USD 2.10 billion in 2023, expected to reach USD 4.08 billion by 2033. The treatment segment represents a smaller share, emphasizing the vaccine's role in preventive healthcare.
23valent Pneumococcal Polysaccharide Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in 23valent Pneumococcal Polysaccharide Vaccine Industry
Pfizer Inc.:
Pfizer is noted for its extensive research in vaccines, including the PPSV23, contributing to public health with innovative solutions that address global needs.Merck & Co., Inc.:
Merck is known for its commitment to vaccine development and production, leading in efforts to enhance community health through improved vaccination access.Sanofi Pasteur:
Sanofi Pasteur focuses on advancing vaccination strategies globally, emphasizing research and development to strengthen their vaccine portfolio.GlaxoSmithKline plc:
GSK plays a significant role in vaccine development, including pneumococcal vaccines, dedicated to ensuring equitable access to vaccines worldwide.We're grateful to work with incredible clients.









FAQs
What is the market size of 23valent Pneumococcal Polysaccharide Vaccine?
The global market size for the 23valent pneumococcal polysaccharide vaccine is projected to reach $2.5 billion by 2033, growing at a CAGR of 6.7% from its initial valuation in 2023.
What are the key market players or companies in this 23valent Pneumococcal Polysaccharide Vaccine industry?
Key players in the 23valent pneumococcal polysaccharide vaccine market include GlaxoSmithKline, Merck & Co., Pfizer Inc., and Sanofi Pasteur, among others, driving innovative solutions and collaboration within the industry.
What are the primary factors driving the growth in the 23valent Pneumococcal Polysaccharide Vaccine industry?
Primary growth factors include rising pneumococcal disease prevalence, expanded immunization programs, increased healthcare spending, and strong government initiatives targeting prevention of respiratory infections.
Which region is the fastest Growing in the 23valent Pneumococcal Polysaccharide Vaccine?
Currently, North America is the fastest-growing region for the 23valent pneumococcal polysaccharide vaccine market, projected to grow from $0.90 billion in 2023 to $1.76 billion by 2033.
Does ConsaInsights provide customized market report data for the 23valent Pneumococcal Polysaccharide Vaccine industry?
Yes, ConsaInsights offers tailored market reports with specific focus areas, allowing clients to receive customized insights and data that suit their strategic needs in the pneumococcal vaccine market.
What deliverables can I expect from this 23valent Pneumococcal Polysaccharide Vaccine market research project?
Expect comprehensive reports including market forecasts, competitive analysis, regional trends, segment performance, and strategic recommendations for operational and investment decision-making.
What are the market trends of 23valent Pneumococcal Polysaccharide Vaccine?
Market trends include increased adoption of innovative vaccine delivery systems, focus on preventive care, strategic partnerships, and regulatory support promoting vaccine accessibility and education.