Acute Lymphoblastic Leukemia Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: acute-lymphoblastic-leukemia-therapeutics
Acute Lymphoblastic Leukemia Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Acute Lymphoblastic Leukemia (ALL) therapeutics market from 2023 to 2033, including market size, growth rates, and insights into key segments and regional performance.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
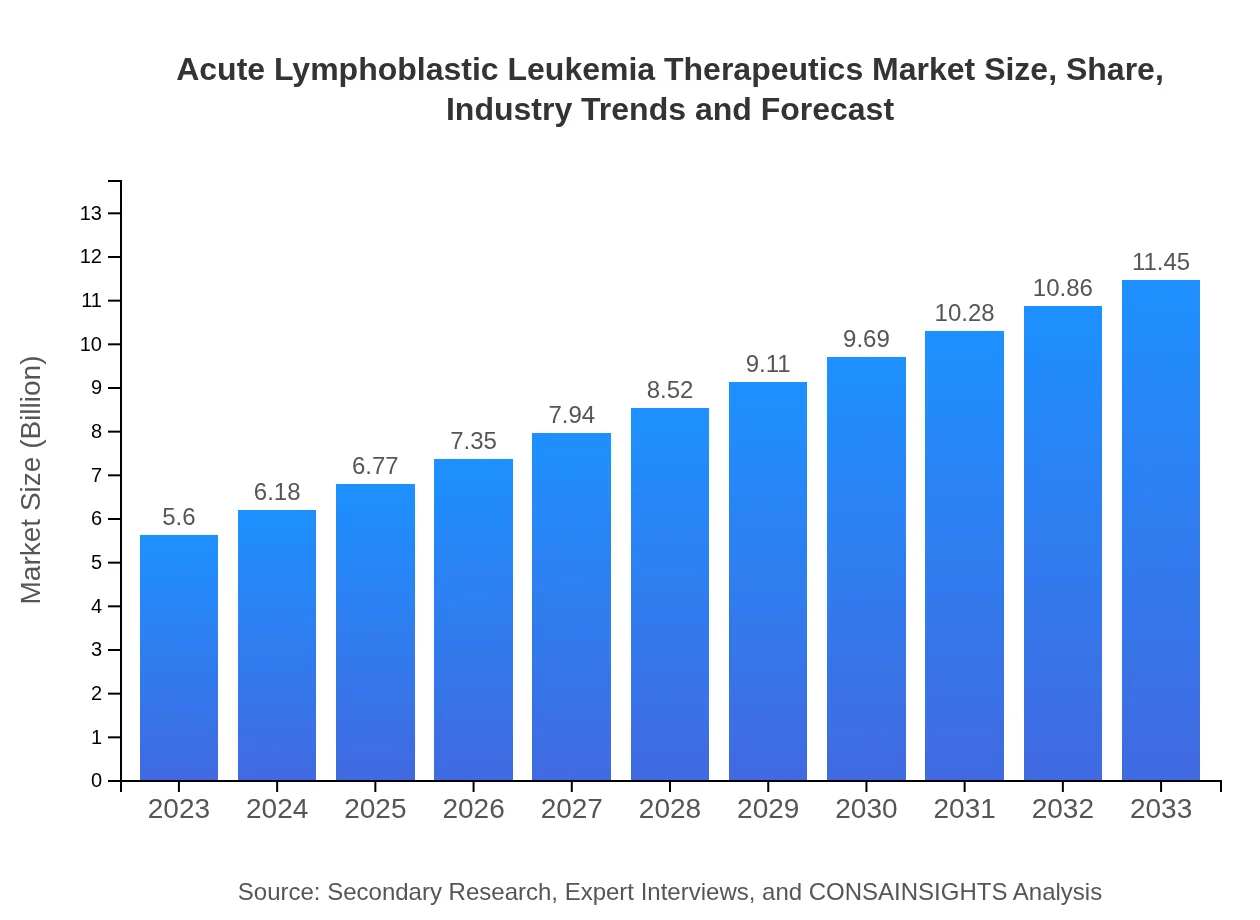
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $11.45 Billion |
| Top Companies | Novartis, Roche, Bristol-Myers Squibb, Gilead Sciences, Amgen |
| Last Modified Date | 31 January 2026 |
Acute Lymphoblastic Leukemia Therapeutics Market Overview
Customize Acute Lymphoblastic Leukemia Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Acute Lymphoblastic Leukemia Therapeutics market size, growth, and forecasts.
- ✔ Understand Acute Lymphoblastic Leukemia Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Acute Lymphoblastic Leukemia Therapeutics
What is the Market Size & CAGR of Acute Lymphoblastic Leukemia Therapeutics market in 2033?
Acute Lymphoblastic Leukemia Therapeutics Industry Analysis
Acute Lymphoblastic Leukemia Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Acute Lymphoblastic Leukemia Therapeutics Market Analysis Report by Region
Europe Acute Lymphoblastic Leukemia Therapeutics Market Report:
Europe's market for Acute Lymphoblastic Leukemia therapeutics will grow from $1.44 billion in 2023 to $2.95 billion by 2033. Initiatives to improve treatment standards and patient outcomes are driving this growth.Asia Pacific Acute Lymphoblastic Leukemia Therapeutics Market Report:
In the Asia Pacific region, the market for Acute Lymphoblastic Leukemia therapeutics is expected to rise from $1.11 billion in 2023 to approximately $2.28 billion by 2033. Factors contributing to this growth include an increasing prevalence of ALL, enhanced healthcare infrastructure, and rising investments in biotechnology.North America Acute Lymphoblastic Leukemia Therapeutics Market Report:
North America, being a leader in the healthcare market, is projected to grow from $2.12 billion in 2023 to around $4.33 billion in 2033. The region benefits from advanced research facilities, strong regulatory frameworks, and significant pharmaceutical investments.South America Acute Lymphoblastic Leukemia Therapeutics Market Report:
South America’s market for ALL therapeutics is anticipated to grow from $0.49 billion in 2023 to $0.99 billion by 2033. The expansion in healthcare access and investment in cancer research underpins the growth in this region, despite challenges such as regulatory hurdles.Middle East & Africa Acute Lymphoblastic Leukemia Therapeutics Market Report:
The Middle East and Africa region's market for ALL therapeutics is likely to increase from $0.44 billion in 2023 to $0.90 billion by 2033. This growth is fueled by improvements in healthcare systems and increased government support for cancer treatment outcomes.Tell us your focus area and get a customized research report.
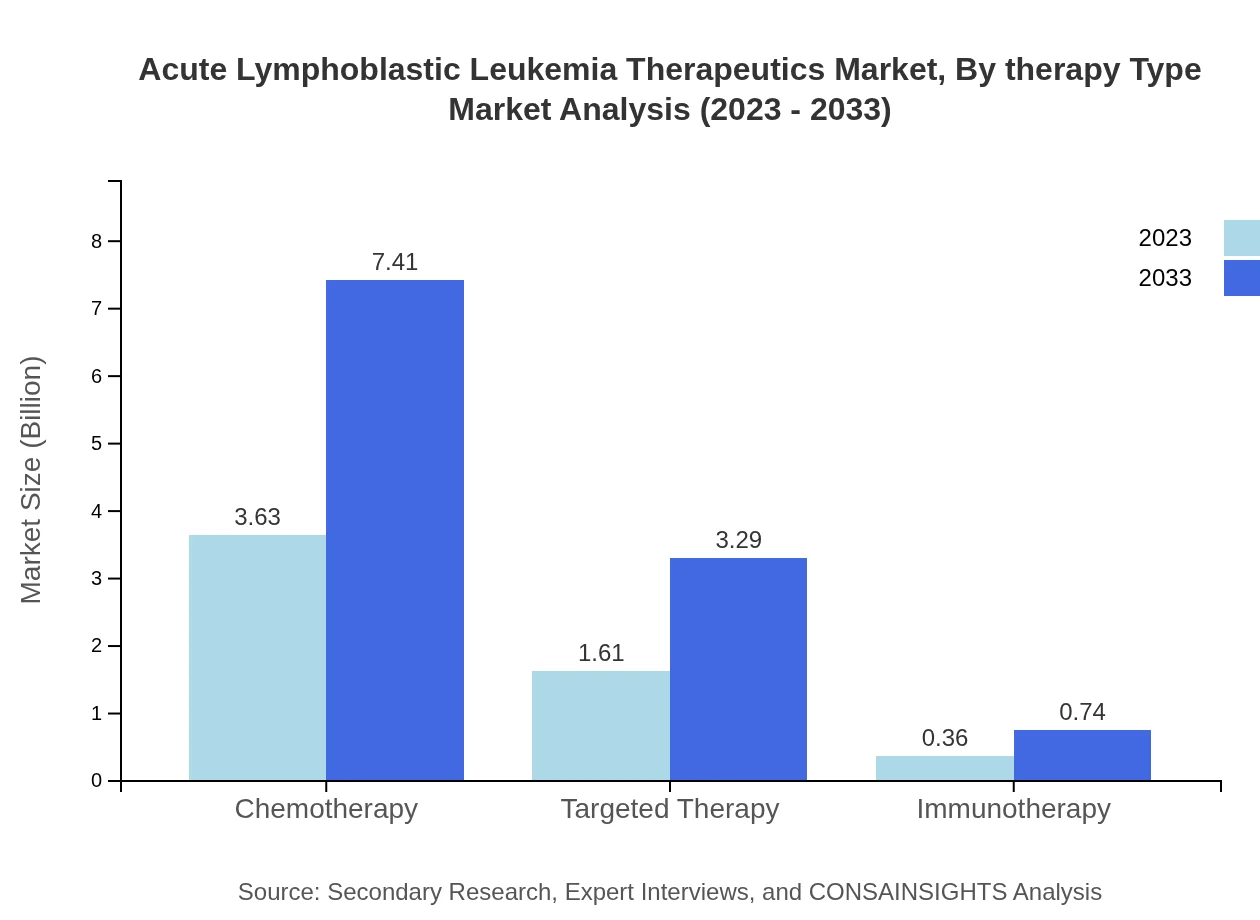
Acute Lymphoblastic Leukemia Therapeutics Market Analysis By Therapy Type
The market for Acute Lymphoblastic Leukemia therapeutics according to therapy types includes chemotherapy, targeted therapy, and immunotherapy. Chemotherapy remains the standard treatment, comprising a substantial portion of the market share at 64.76% in 2023. Targeted therapy accounts for 28.75%, and immunotherapy, although emerging, captures 6.49% in the same year. The market is expected to evolve with increased reliance on novel therapies and combination treatments.
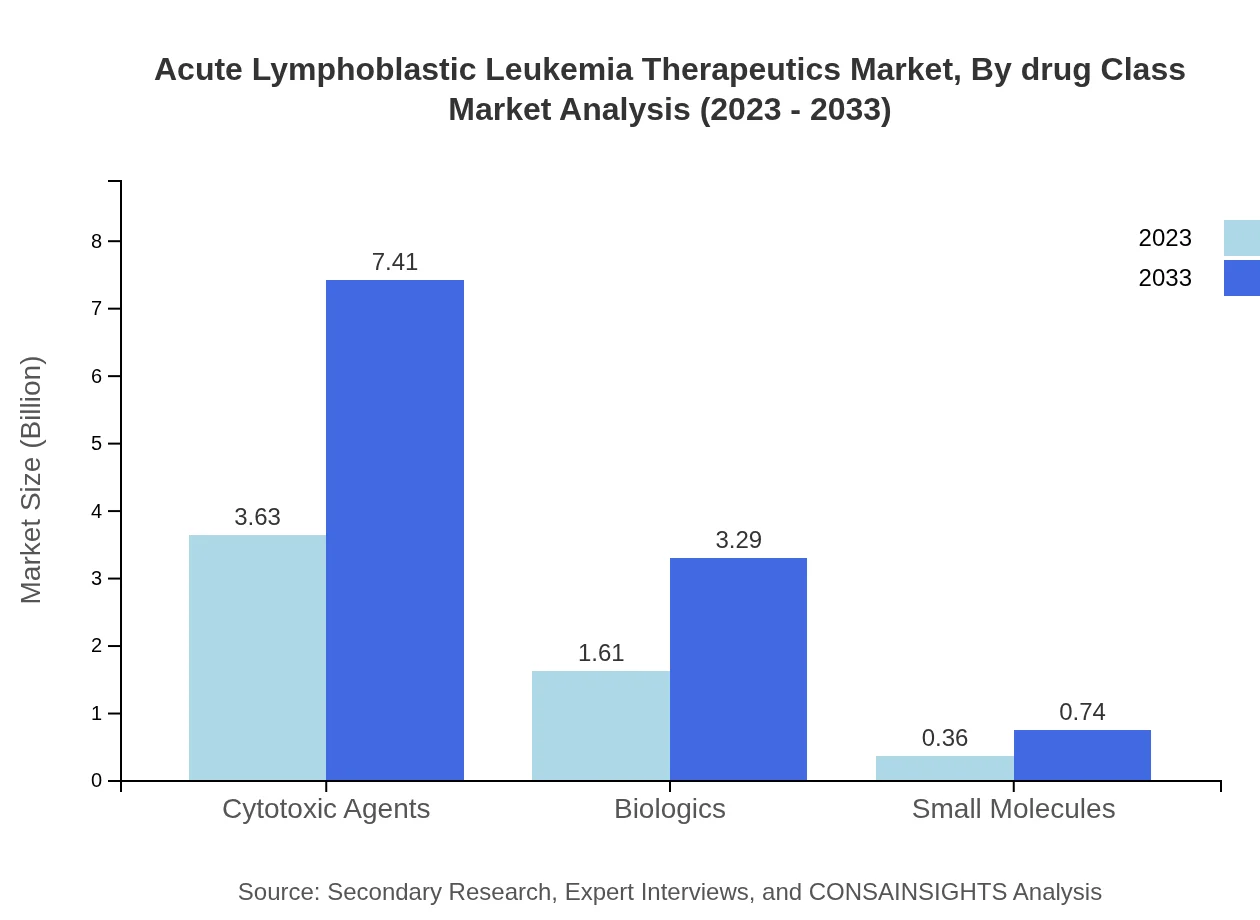
Acute Lymphoblastic Leukemia Therapeutics Market Analysis By Drug Class
Drug classes in the ALL therapeutics market include cytotoxic agents, biologics, and small molecules. Cytotoxic agents occupy the largest market share at 64.76%, while biologics and small molecules hold shares of 28.75% and 6.49% respectively in 2023. The forecast predicts an increased focus on biologics and innovative drugs, pushing the market towards a more diverse product offering.
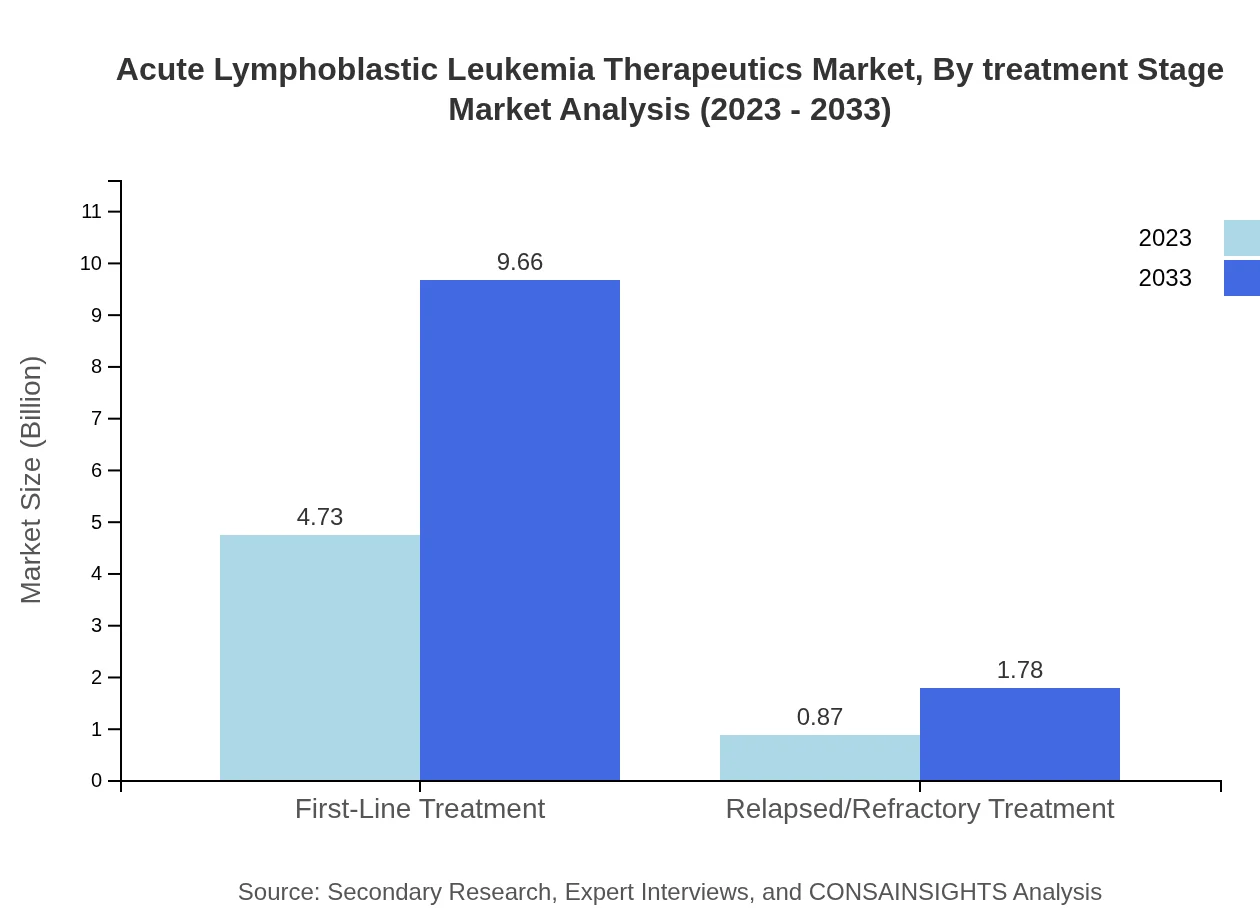
Acute Lymphoblastic Leukemia Therapeutics Market Analysis By Treatment Stage
The treatment stage for Acute Lymphoblastic Leukemia divides into first-line treatment and relapsed/refractory treatment. First-line treatments, which are more common, dominate the market with a share of 84.43%. The focus on early detection and treatment is key to improving outcomes, with a steady growth projected for this segment.
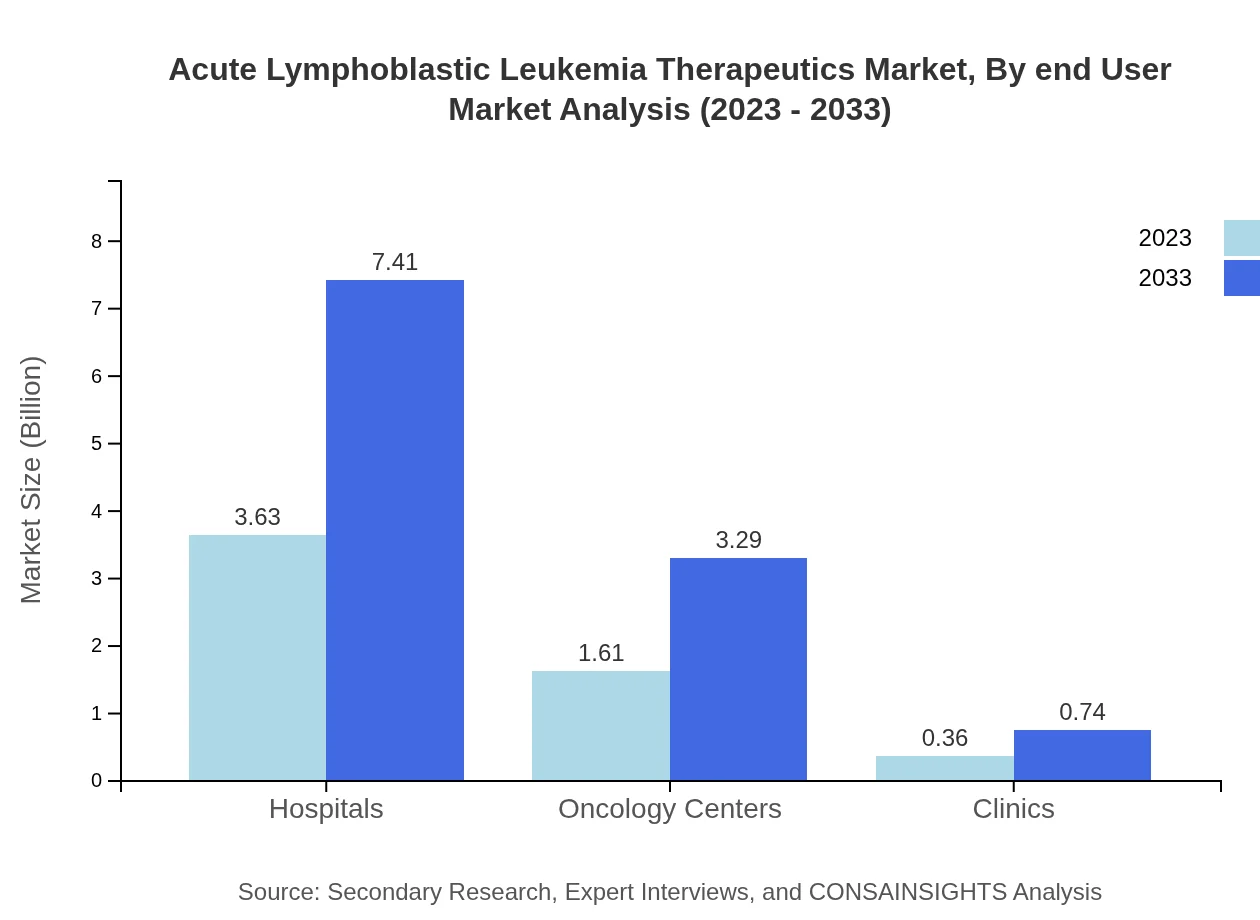
Acute Lymphoblastic Leukemia Therapeutics Market Analysis By End User
End-users of Acute Lymphoblastic Leukemia therapeutics primarily include hospitals, oncology centers, and clinics. Hospitals capture about 64.76% of the market share in 2023, highlighting their critical role in administering treatment. Oncology centers and clinics, while smaller, are growing segments, reflecting a trend towards more specialized care and outpatient services as overall cancer treatment standards improve.
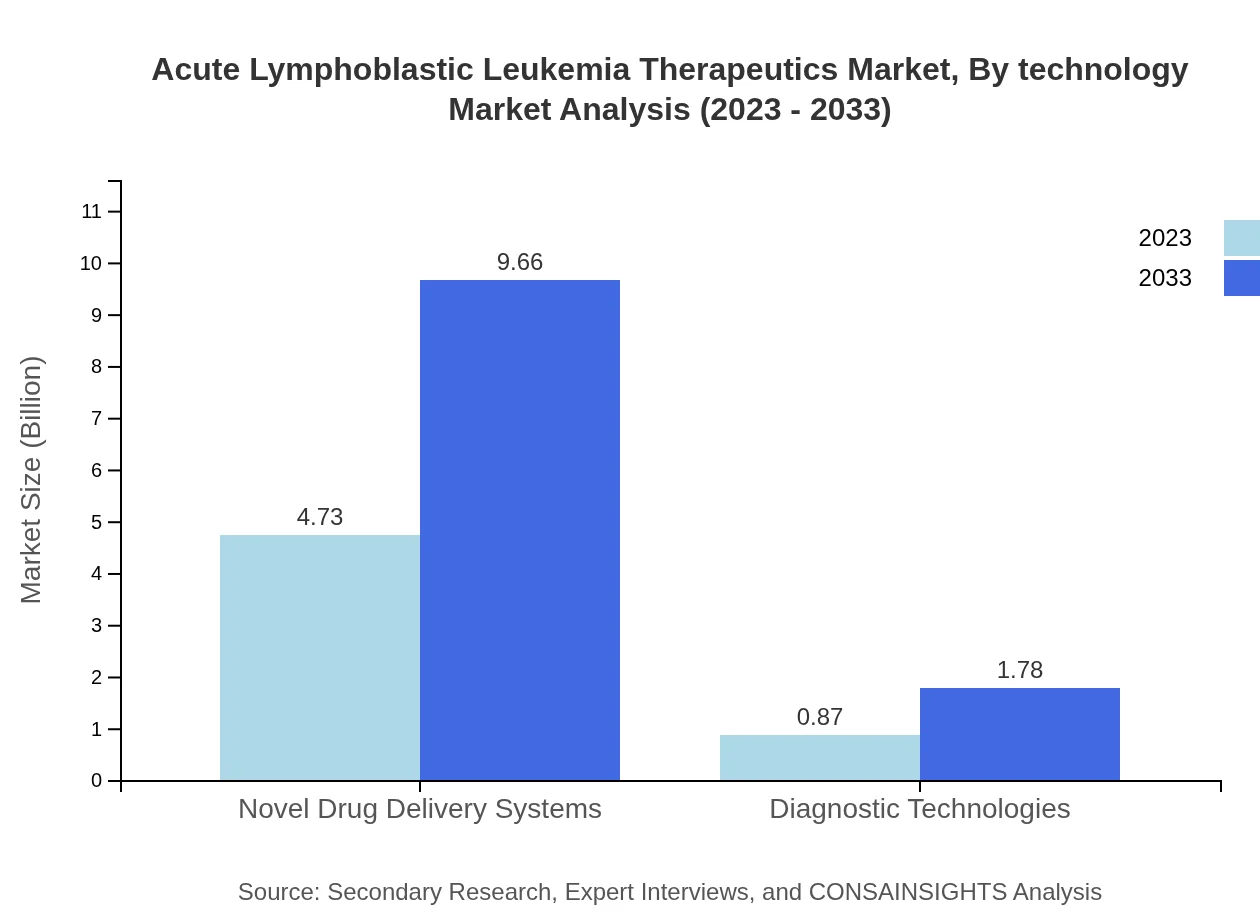
Acute Lymphoblastic Leukemia Therapeutics Market Analysis By Technology
Technological advancements play a pivotal role in the Acute Lymphoblastic Leukemia therapeutics market, particularly in novel drug delivery systems, diagnostic technologies, and treatment monitoring solutions. The rise of innovative delivery systems, accounting for 84.43% of the market share in 2023, highlights the need for efficient administration and personalized treatment strategies.
Acute Lymphoblastic Leukemia Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Acute Lymphoblastic Leukemia Therapeutics Industry
Novartis:
Novartis is a leading global healthcare company that focuses on developing treatments for various cancers, including advanced therapies for ALL patients. Their innovative work in CAR-T cell therapy has transformed ALL treatment.Roche:
Roche is a pioneer in biotechnology and oncology, providing notable drugs that target ALL. Their commitment to research and development emphasizes precision medicine and comprehensive patient care.Bristol-Myers Squibb:
Bristol-Myers Squibb specializes in biopharma solutions with multiple approved therapies for ALL. Their innovative approaches to drug design cater to high-risk patient populations.Gilead Sciences:
Gilead Sciences focuses on developing transformative medicines for patients with cancer. Their research in immune-oncology is paving the way for novel treatment options for ALL.Amgen:
Amgen is dedicated to discovering and developing innovative medicines. Their approach integrates biology and technology to develop therapies for ALL.We're grateful to work with incredible clients.









FAQs
What is the market size of acute Lymphoblastic Leukemia Therapeutics?
The acute lymphoblastic leukemia therapeutics market is valued at approximately $5.6 billion in 2023, with a projected CAGR of 7.2% through 2033. This growth reflects increasing demand for innovative therapies and treatment options for patients globally.
What are the key market players or companies in this acute Lymphoblastic Leukemia Therapeutics industry?
Key players in the acute lymphoblastic leukemia therapeutics market include major pharmaceutical companies engaged in oncology. These companies are continually investing in R&D to enhance treatment efficacy and expand their portfolios with novel therapeutic agents.
What are the primary factors driving the growth in the acute Lymphoblastic Leukemia Therapeutics industry?
Driving factors include an increasing incidence of acute lymphoblastic leukemia, advancements in treatment technologies, and the rising demand for personalized medicine. Additionally, government initiatives to improve cancer care accessibility contribute to market growth.
Which region is the fastest Growing in the acute Lymphoblastic Leukemia Therapeutics?
The Asia Pacific region is the fastest-growing market for acute lymphoblastic leukemia therapeutics, expected to expand from $1.11 billion in 2023 to $2.28 billion by 2033, backed by improving healthcare infrastructure and rising investments in oncology treatments.
Does ConsaInsights provide customized market report data for the acute Lymphoblastic Leukemia Therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs, enabling stakeholders to gain detailed insights relevant to the acute lymphoblastic leukemia therapeutics industry, catering to various research and analytical requirements.
What deliverables can I expect from this acute Lymphoblastic Leukemia Therapeutics market research project?
Deliverables encompass comprehensive reports, market size analyses, growth forecasts, competitive landscape evaluations, and segment-wise insights that collectively provide a detailed overview of trends and dynamics in the acute lymphoblastic leukemia therapeutics market.
What are the market trends of acute Lymphoblastic Leukemia Therapeutics?
Market trends include a surge in biologics and novel drug delivery systems, which are increasingly favored due to their targeted efficacy. Additionally, the focus on first-line treatments and personalized therapies is shaping the future of this market segment.