Adalimumab Drugs Market Report
Published Date: 31 January 2026 | Report Code: adalimumab-drugs
Adalimumab Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Adalimumab Drugs market, including insights into market size, segmentation, regional trends, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
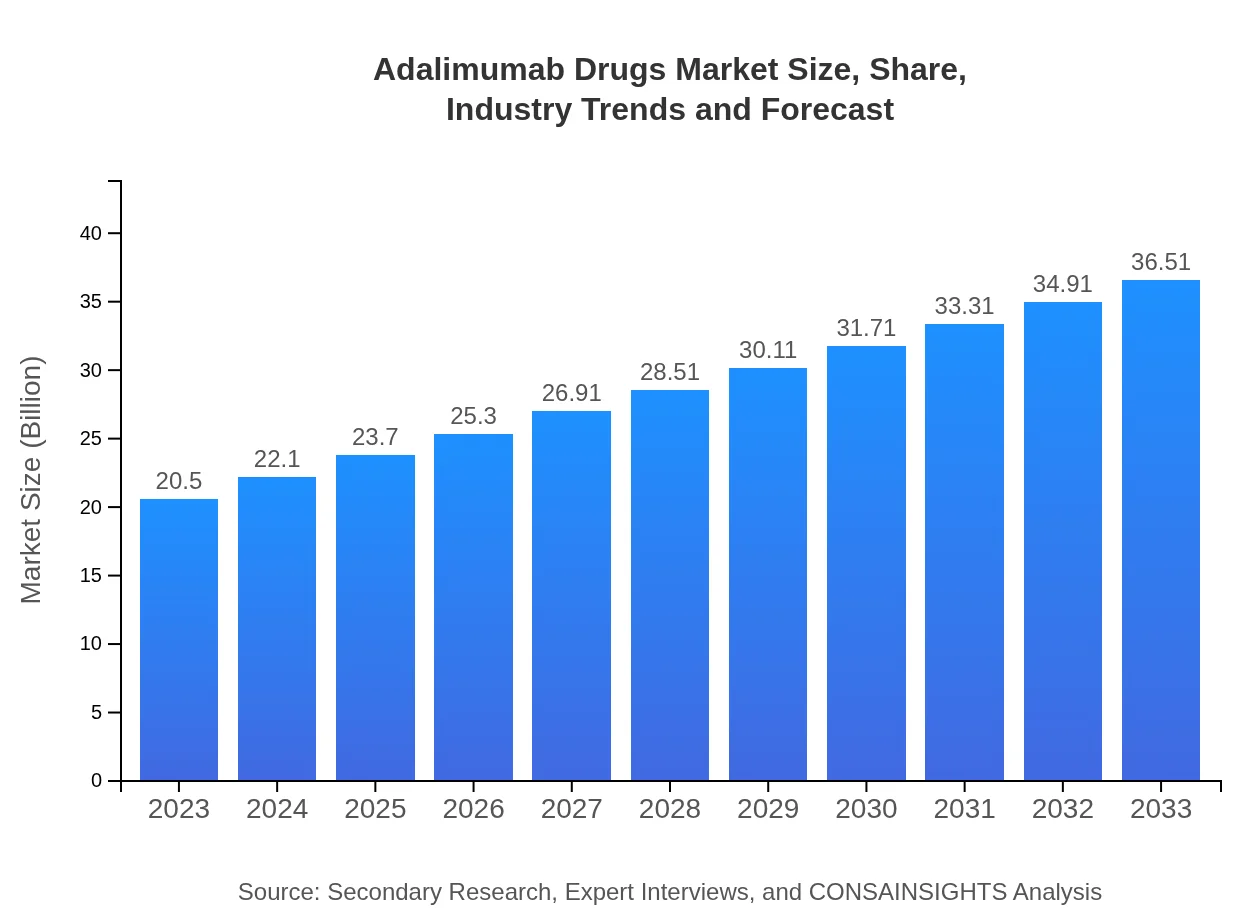
| 2023 Market Size | $20.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $36.51 Billion |
| Top Companies | AbbVie Inc., Amgen Inc., Samsung Bioepis, Mylan N.V. |
| Last Modified Date | 31 January 2026 |
Adalimumab Drugs Market Overview
Customize Adalimumab Drugs Market Report market research report
- ✔ Get in-depth analysis of Adalimumab Drugs market size, growth, and forecasts.
- ✔ Understand Adalimumab Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Adalimumab Drugs
What is the Market Size & CAGR of Adalimumab Drugs market in 2023?
Adalimumab Drugs Industry Analysis
Adalimumab Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Adalimumab Drugs Market Analysis Report by Region
Europe Adalimumab Drugs Market Report:
Europe's market will expand from $5.88 billion in 2023 to $10.48 billion by 2033. The presence of advanced healthcare systems, along with stringent regulatory approvals for biosimilars, continues to drive market growth.Asia Pacific Adalimumab Drugs Market Report:
In the Asia Pacific, the Adalimumab Drugs market size is projected to grow from $4.13 billion in 2023 to $7.35 billion by 2033. Factors such as increasing healthcare access, rising incidences of autoimmune disorders, and growing investment in healthcare infrastructure are driving this growth.North America Adalimumab Drugs Market Report:
The North American market is the largest, expected to grow from $6.74 billion in 2023 to $12.01 billion by 2033. The region benefits from well-established healthcare systems, high healthcare expenditure, and rapid adoption of innovative treatments.South America Adalimumab Drugs Market Report:
South America's Adalimumab market is expected to see growth from $1.77 billion in 2023 to $3.14 billion by 2033. The expanding healthcare access and increasing awareness regarding efficacious treatments are pivotal for market expansion in this region.Middle East & Africa Adalimumab Drugs Market Report:
The Middle East and Africa market is anticipated to grow from $1.98 billion in 2023 to $3.53 billion by 2033. Initiatives to enhance healthcare delivery and increased pharmaceutical penetration are crucial for market advancement here.Tell us your focus area and get a customized research report.
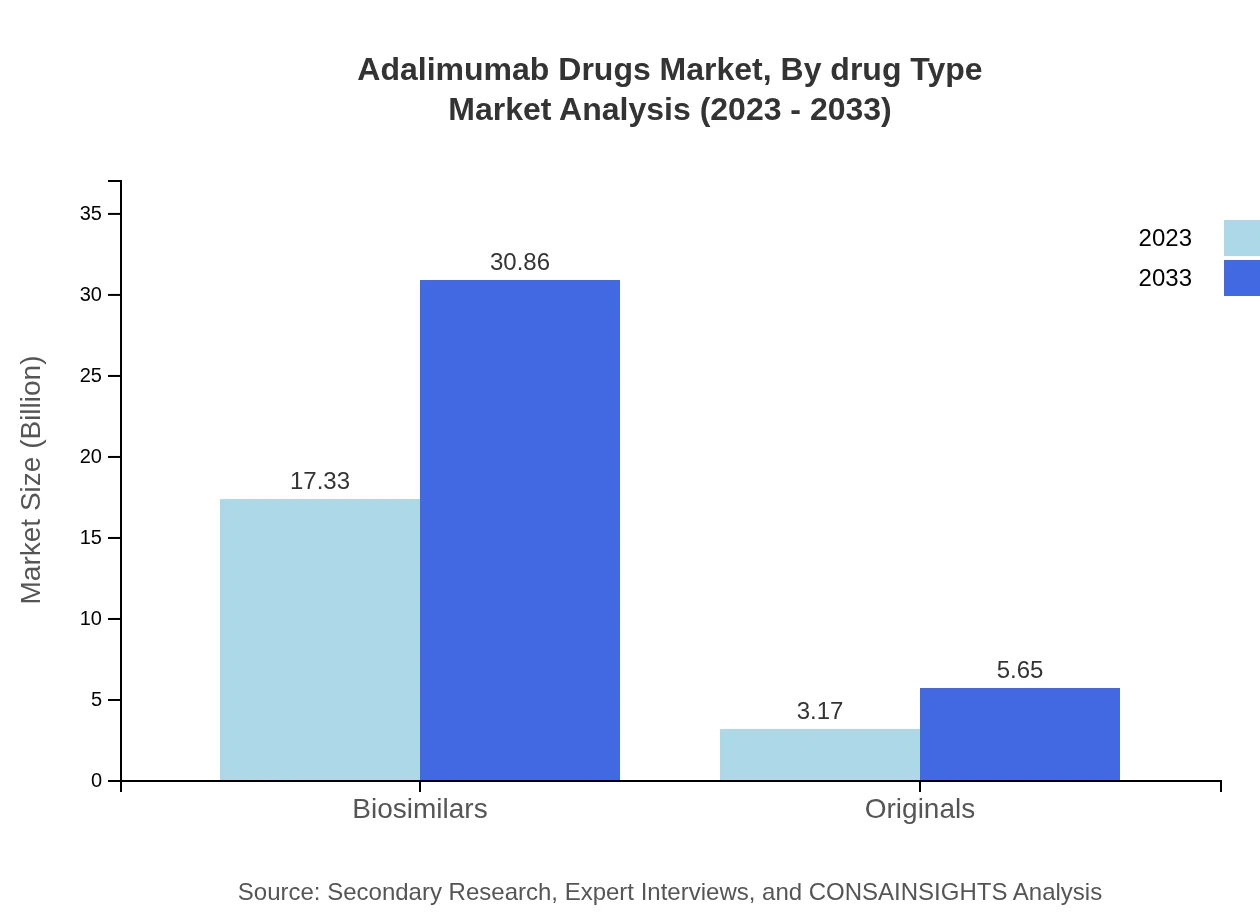
Adalimumab Drugs Market Analysis By Drug Type
The segment comprising Biosimilars values $17.33 billion in 2023 and is projected to reach $30.86 billion by 2033, maintaining an 84.52% market share. Originals currently account for $3.17 billion, expected to grow to $5.65 billion, with a market share of 15.48%.
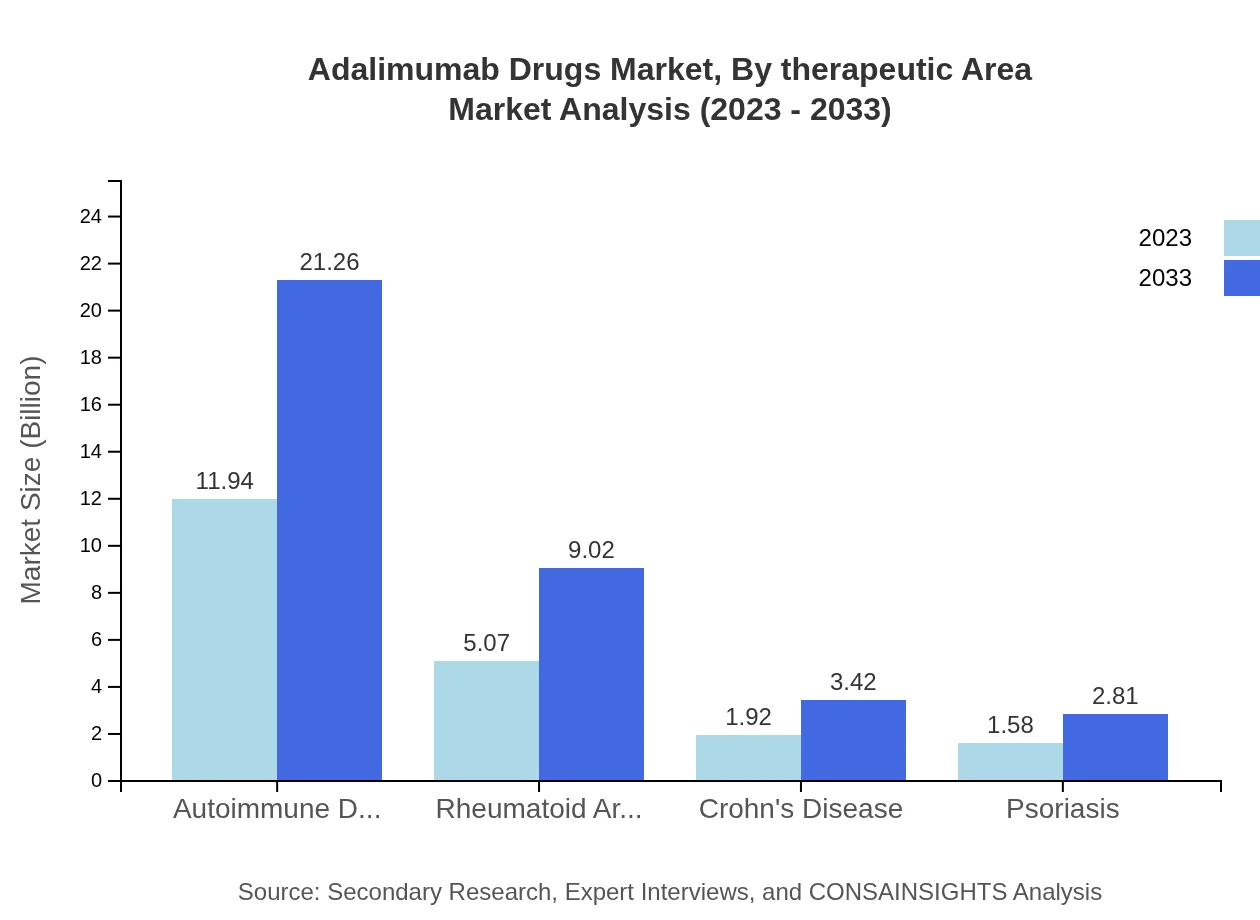
Adalimumab Drugs Market Analysis By Therapeutic Area
The Autoimmune Disorders segment is significant, growing from $11.94 billion in 2023 to $21.26 billion by 2033, dominating by holding a share of 58.23%. Notable growth is also expected in Rheumatoid Arthritis and Crohn's Disease categories.
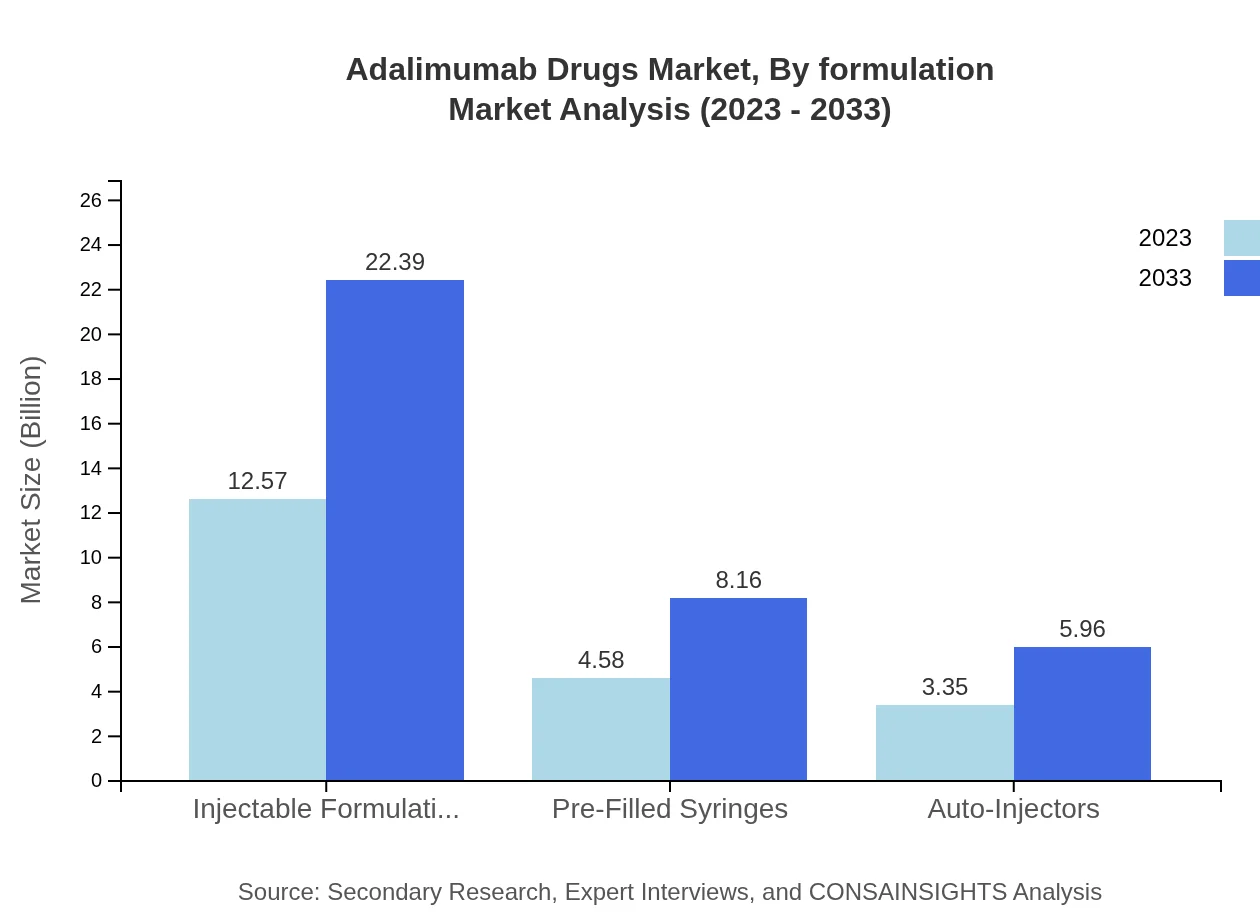
Adalimumab Drugs Market Analysis By Formulation
Injectable formulations generate $12.57 billion, projected to grow to $22.39 billion, holding a 61.31% market share. Pre-Filled Syringes and Auto-Injectors provide alternative methods of administration, with shares of 22.36% and 16.33%, respectively.
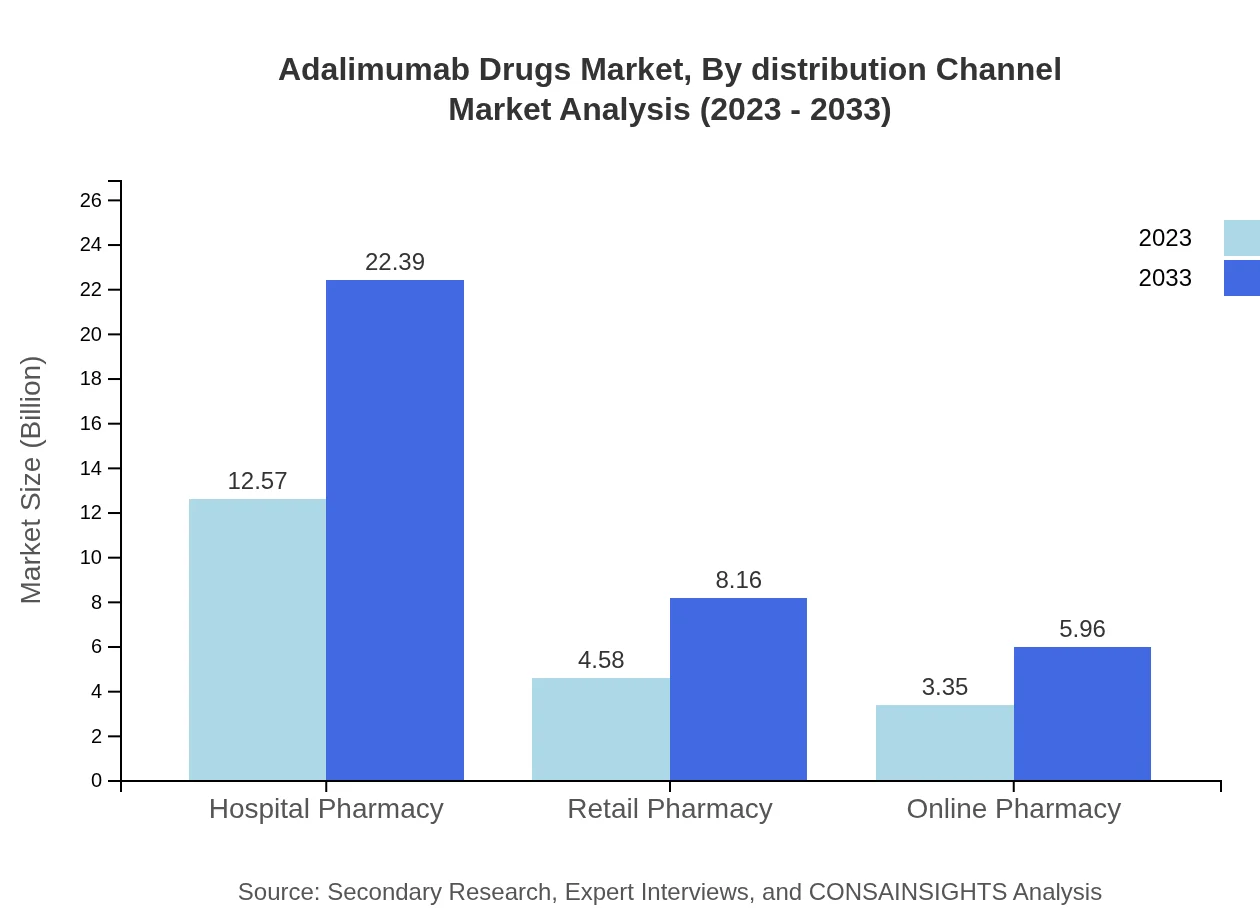
Adalimumab Drugs Market Analysis By Distribution Channel
Hospital pharmacies dominate the distribution channel, with $12.57 billion in 2023 and set to reach $22.39 billion, maintaining a 61.31% market share. Retail and online pharmacies also contribute significantly, holding respective shares of 22.36% and 16.33%.
Adalimumab Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Adalimumab Drugs Industry
AbbVie Inc.:
The original developer of Adalimumab, AbbVie Inc. leads the market with its innovative therapies and strong patent portfolio.Amgen Inc.:
Amgen is a prominent player in the biosimilars segment, contributing to increased market competition by offering affordable alternatives to original formulations.Samsung Bioepis:
This company has rapidly established itself in the biosimilars sector with its high-quality and cost-effective Adalimumab biosimilar.Mylan N.V.:
Mylan is notable for bringing generic versions of Adalimumab to various emerging markets, enhancing market accessibility.We're grateful to work with incredible clients.









FAQs
What is the market size of adalimumab Drugs?
The global adalimumab drugs market was valued at approximately $20.5 billion in 2023, with a projected CAGR of 5.8% leading up to 2033. This indicates significant ongoing growth within this pharmaceutical segment, providing opportunities for investment and expansion.
What are the key market players or companies in this adalimumab Drugs industry?
Key players in the adalimumab drug market include major pharmaceutical companies such as AbbVie, Amgen, and Samsung Bioepis. These companies are pivotal in driving innovation, developing biosimilars, and maintaining competitive pricing strategies.
What are the primary factors driving the growth in the adalimumab drug industry?
Growth in the adalimumab market is primarily driven by the increasing prevalence of autoimmune diseases, advancements in biosimilar technologies, and the rising demand for effective chronic disease management solutions. Additionally, expanding healthcare access boosts market dynamics.
Which region is the fastest Growing in the adalimumab drug market?
The North American region is the fastest-growing in the adalimumab drug market, projected to expand from $6.74 billion in 2023 to $12.01 billion by 2033. Significant advancements in healthcare infrastructure and rising awareness contribute to this rapid growth.
Does ConsaInsights provide customized market report data for the adalimumab drug industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the adalimumab drug industry. Clients can request in-depth analyses and market forecasts designed to meet their strategic objectives and investment criteria.
What deliverables can I expect from this adalimumab drug market research project?
Expect comprehensive deliverables from the adalimumab drug market research project, including detailed market analyses, segmentation data, competitive landscape insights, and growth forecasts tailored to your specifications, ensuring informed decision-making.
What are the market trends of adalimumab drugs?
Market trends in adalimumab drugs reflect a shift towards biosimilars and innovative drug delivery methods. Notably, the biosimilars segment is growing significantly, projected to reach $30.86 billion by 2033, highlighting a crucial industry transition.