Adme Toxicology Testing Market Report
Published Date: 31 January 2026 | Report Code: adme-toxicology-testing
Adme Toxicology Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Adme Toxicology Testing market, including market size, trends, and forecasts for 2023-2033. Insights into segmentation, regional dynamics, and key players are also included for strategic planning.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
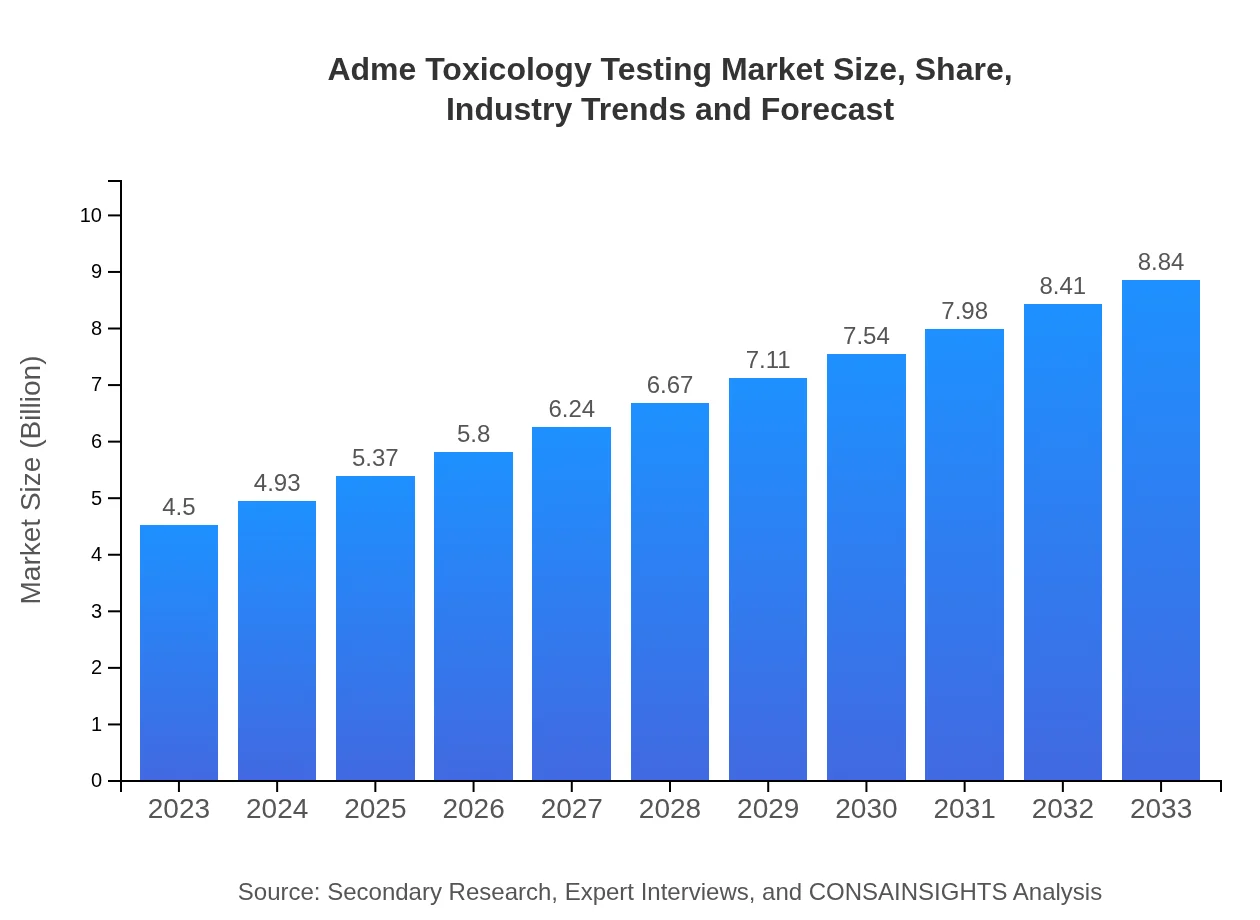
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $8.84 Billion |
| Top Companies | Charles River Laboratories, Covance Inc., Charles River Laboratories, Envigo, WuXi AppTec |
| Last Modified Date | 31 January 2026 |
Adme Toxicology Testing Market Overview
Customize Adme Toxicology Testing Market Report market research report
- ✔ Get in-depth analysis of Adme Toxicology Testing market size, growth, and forecasts.
- ✔ Understand Adme Toxicology Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Adme Toxicology Testing
What is the Market Size & CAGR of Adme Toxicology Testing market in 2023 and 2033?
Adme Toxicology Testing Industry Analysis
Adme Toxicology Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Adme Toxicology Testing Market Analysis Report by Region
Europe Adme Toxicology Testing Market Report:
The European market for Adme Toxicology Testing is expected to increase from $1.25 billion in 2023 to $2.45 billion by 2033. The region benefits from stringent regulations that mandate thorough safety testing for pharmaceuticals, coupled with a large number of biotech and pharmaceutical companies focused on drug development. Collaborative frameworks among industry players and research institutions further bolster the market.Asia Pacific Adme Toxicology Testing Market Report:
The Asia Pacific region is emerging as a significant player in the Adme Toxicology Testing market, valued at approximately $0.96 billion in 2023 and projected to reach $1.88 billion by 2033. This growth is driven by increased investments in research and development, alongside collaborations between local CROs and multinational pharmaceutical companies. Enhanced regulatory support and the growing biotech landscape further bolster market potential.North America Adme Toxicology Testing Market Report:
North America remains the largest market for Adme Toxicology Testing, projected to grow from $1.54 billion in 2023 to $3.02 billion by 2033. Factors driving this growth include the presence of major pharmaceutical firms, advanced research facilities, and a strong emphasis on safety in drug development and regulatory compliance. Continuous innovation in testing technologies also enhances market competitiveness in this region.South America Adme Toxicology Testing Market Report:
In South America, the Adme Toxicology Testing market is expected to grow from $0.22 billion in 2023 to $0.43 billion by 2033. The expansion is primarily attributed to increasing healthcare spending and greater alignment with international testing standards, stimulating demand for toxicology testing services across the region.Middle East & Africa Adme Toxicology Testing Market Report:
In the Middle East and Africa, the market is anticipated to grow from $0.54 billion in 2023 to $1.06 billion by 2033. The region is witnessing an uptick in research activities and improvements in healthcare infrastructure, fueling demand for toxicology testing services. Government initiatives aimed at enhancing biotechnology capabilities also play a pivotal role in market expansion.Tell us your focus area and get a customized research report.
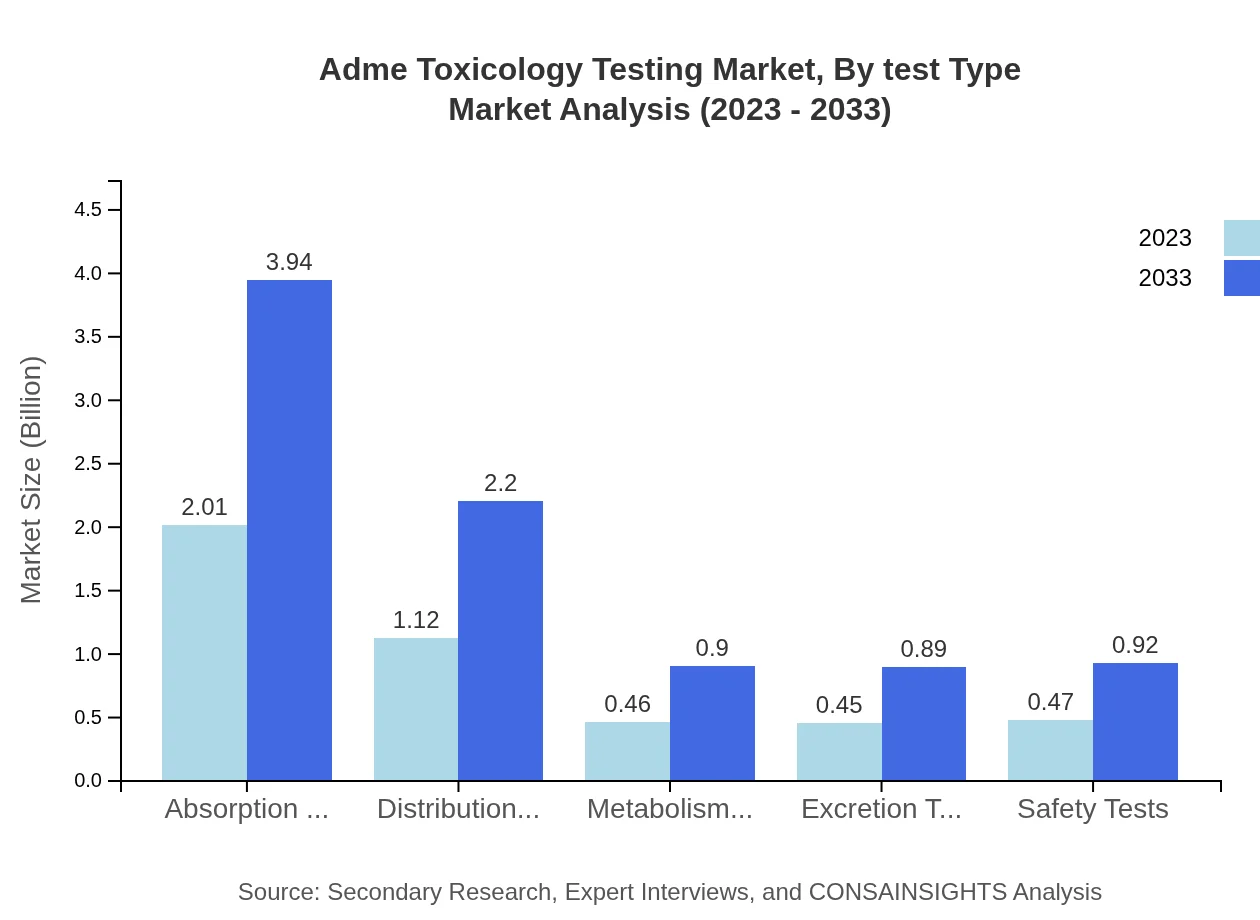
Adme Toxicology Testing Market Analysis By Test Type
The testing type segment includes various tests such as absorption, distribution, metabolism, excretion, and safety tests. In 2023, absorption tests dominate the market at approximately $2.01 billion, accounting for 44.57% market share. Ongoing advancements and demand for more effective absorption methodologies indicate a sustained focus on enhancing this segment's capabilities.
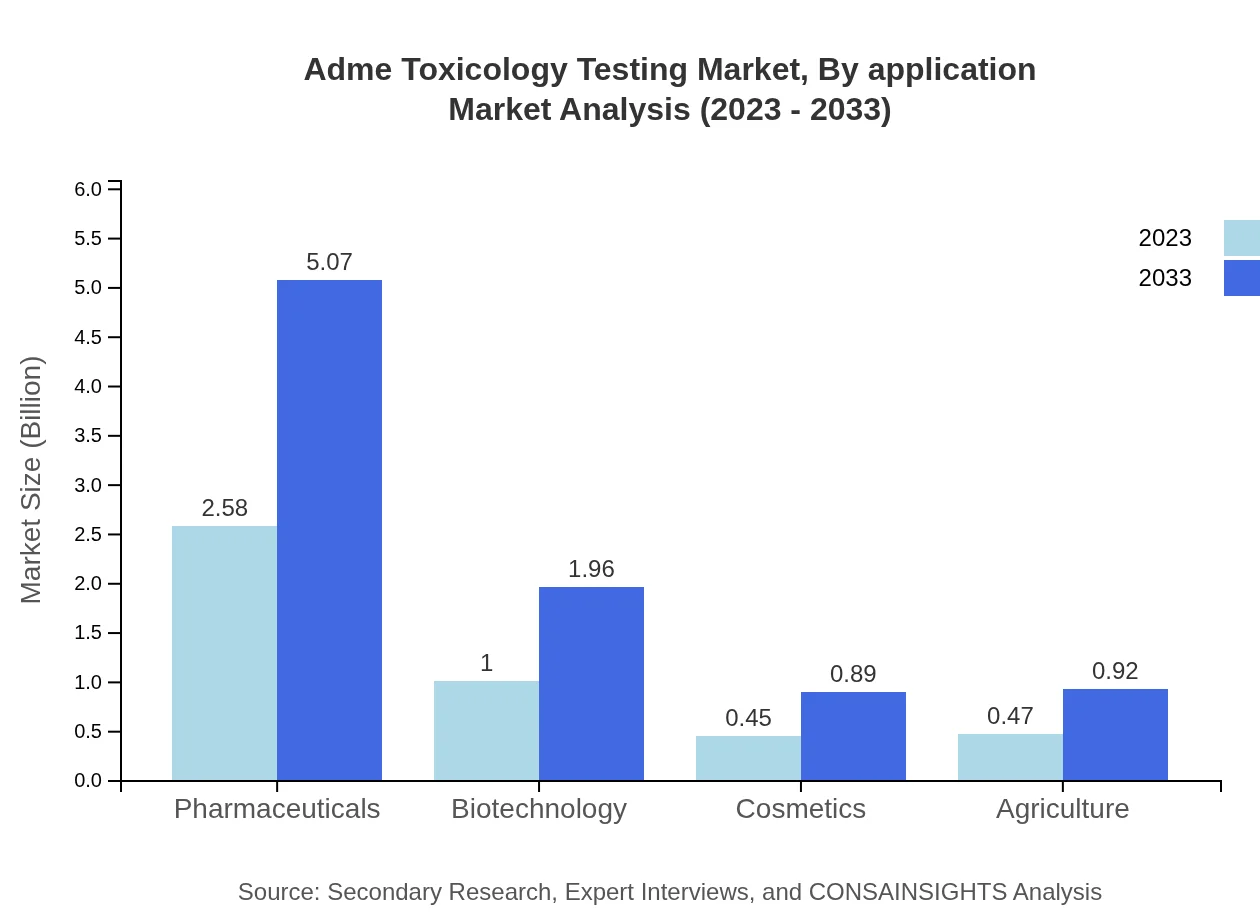
Adme Toxicology Testing Market Analysis By Application
In terms of application, pharmaceutical companies comprise the largest share of the market. As of 2023, they are valued at $2.58 billion, making up 57.33% of the total. The increasing demand for pharmaceuticals and rigorous testing requirements are driving this sector's growth.
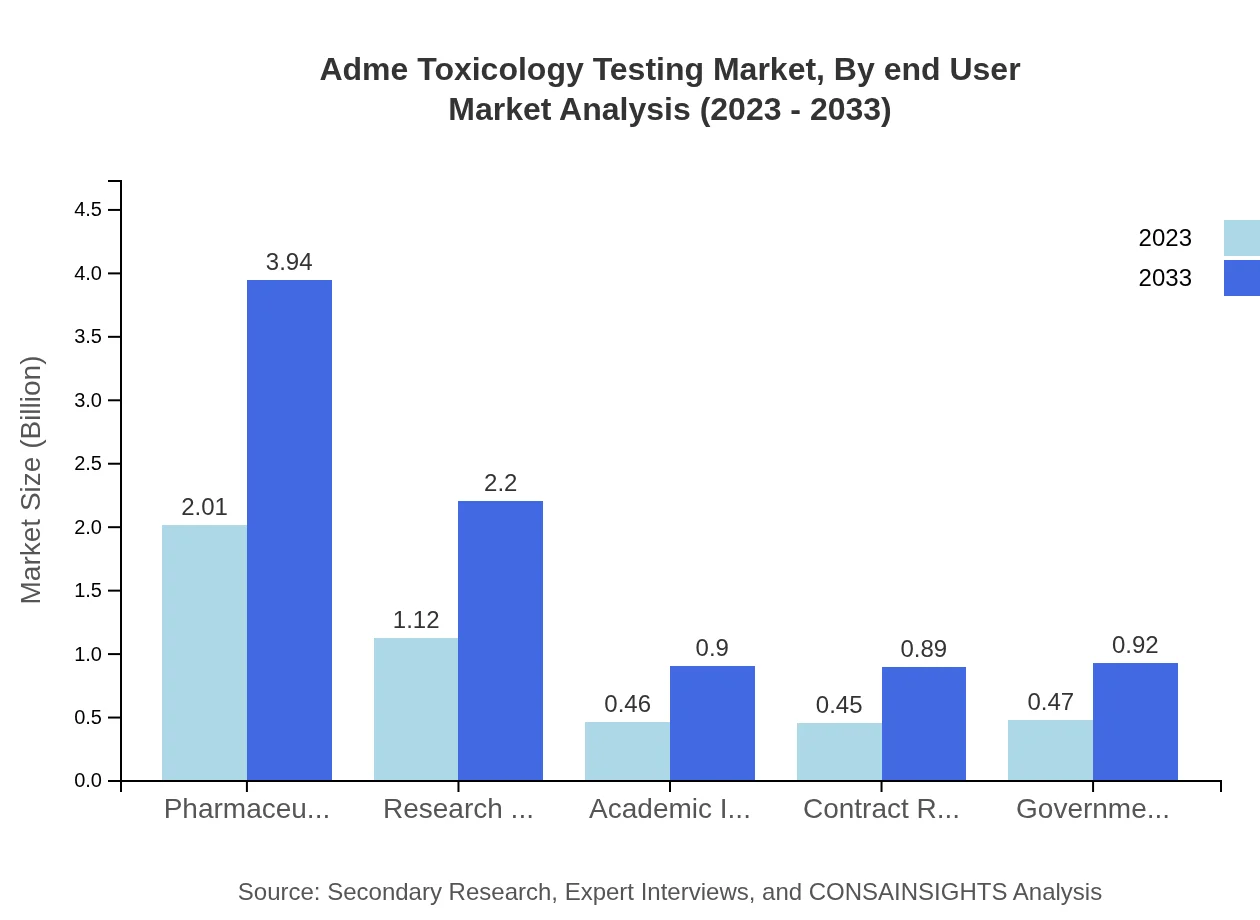
Adme Toxicology Testing Market Analysis By End User
The end-user segmentation showcases diverse categories, including pharmaceutical companies, research organizations, and academic institutions. With pharmaceutical companies leading at 44.57% market share in 2023, the demand from research organizations and academic institutions is also notable, growing in response to evolving industry requirements and collaborative research initiatives.
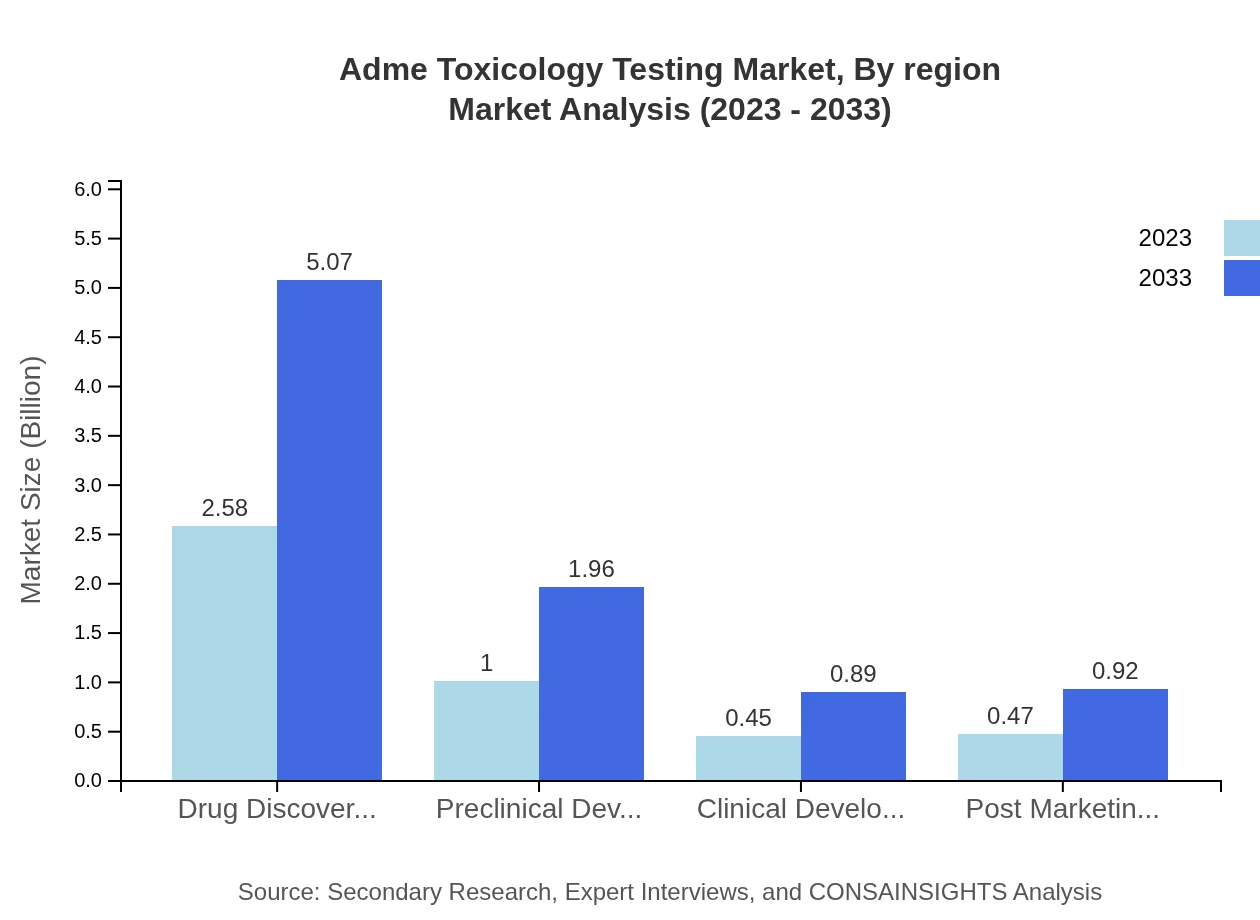
Adme Toxicology Testing Market Analysis By Region
The process phase segment focuses on the critical stages of drug development. The drug discovery phase holds a significant share, valued at $2.58 billion in 2023. This necessitates extensive testing to ensure safety and efficacy, underscoring the importance of this phase in the broader drug development process.
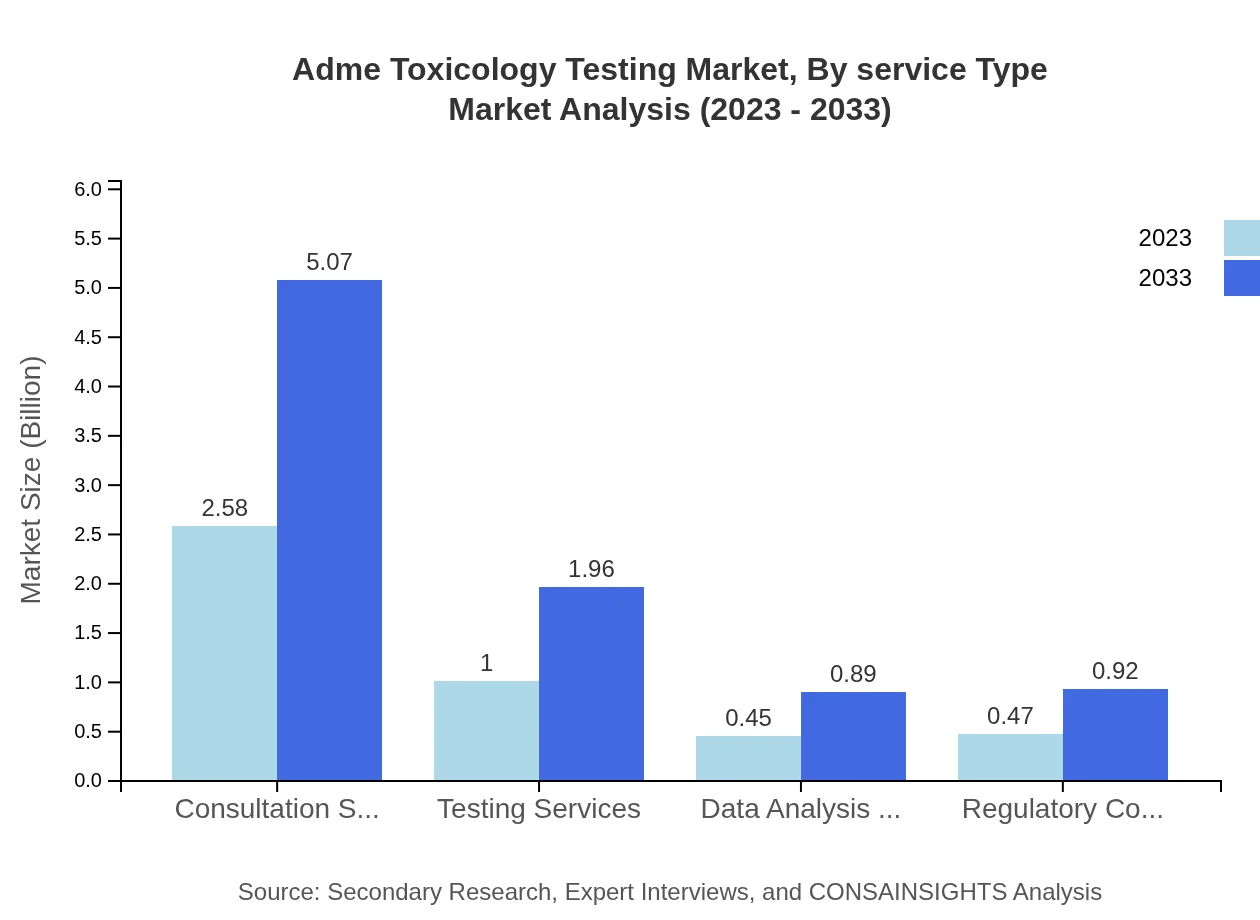
Adme Toxicology Testing Market Analysis By Service Type
Key service types include consultation services, testing services, and regulatory compliance services. Consultation services lead the market with a value of $2.58 billion, driven by the expertise required in navigating complex regulatory landscapes and ensuring compliance in drug development.
Adme Toxicology Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Adme Toxicology Testing Industry
Charles River Laboratories:
A leading global contract research organization (CRO) offering comprehensive services for preclinical and clinical drug development, including toxicology testing.Covance Inc.:
Part of Labcorp, Covance provides drug development solutions tailored to pharmaceuticals and biotechnology, with an emphasis on toxicology testing.Charles River Laboratories:
A key player in the toxicology testing industry, providing extensive research services aimed at accelerating the drug development process for biopharmaceutical companies.Envigo:
Focused on providing research services, Envigo also specializes in delivering essential toxicology testing services to support drug development.WuXi AppTec:
A comprehensive global platform for the R&D and manufacturing of healthcare products offering robust toxicology and ADME testing services.We're grateful to work with incredible clients.









FAQs
What is the market size of adme Toxicology Testing?
The global Adme Toxicology Testing market is valued at approximately $4.5 billion as of 2023, with a projected CAGR of 6.8% over the next decade. By 2033, the market is expected to grow significantly, reflecting increased demand for drug safety and efficacy.
What are the key market players or companies in this adme Toxicology Testing industry?
Key players in the Adme Toxicology Testing market include large pharmaceutical companies such as Pfizer, Johnson & Johnson, and Roche, along with contract research organizations like Covance and Charles River, which contribute significantly to research and development efforts.
What are the primary factors driving the growth in the adme Toxicology Testing industry?
The growth in the Adme Toxicology Testing industry is driven by advancements in drug development technologies, rising R&D investments by pharmaceutical companies, stringent regulatory requirements, and the increasing focus on drug safety and efficacy, alongside an expanding pipeline of new drugs.
Which region is the fastest Growing in the adme Toxicology Testing?
North America is currently the fastest-growing region, with its market size increasing from $1.54 billion in 2023 to an expected $3.02 billion by 2033. This growth is fueled by high R&D expenditure and advancements in biopharmaceutical research.
Does ConsaInsights provide customized market report data for the adme Toxicology Testing industry?
Yes, ConsaInsights offers tailored market report data for the Adme Toxicology Testing industry. Clients can request customized reports based on specific requirements, including geographical focus, market trends, and competitor analysis.
What deliverables can I expect from this adme Toxicology Testing market research project?
From the Adme Toxicology Testing market research project, clients can expect comprehensive reports featuring market size, growth forecasts, segmentation analysis, competitive landscape assessment, and actionable insights tailored to their strategic needs.
What are the market trends of adme Toxicology Testing?
Current market trends in Adme Toxicology Testing include increased adoption of in-vitro testing methods, growing emphasis on personalized medicine, integration of AI and machine learning in toxicological studies, and a shift toward early phase drug development testing to reduce late-stage failures.