Airway Stent Lung Stent Market Report
Published Date: 31 January 2026 | Report Code: airway-stent-lung-stent
Airway Stent Lung Stent Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Airway Stent Lung Stent market from 2023 to 2033, detailing market trends, size, regional insights, industry analysis, and forecast data to guide stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
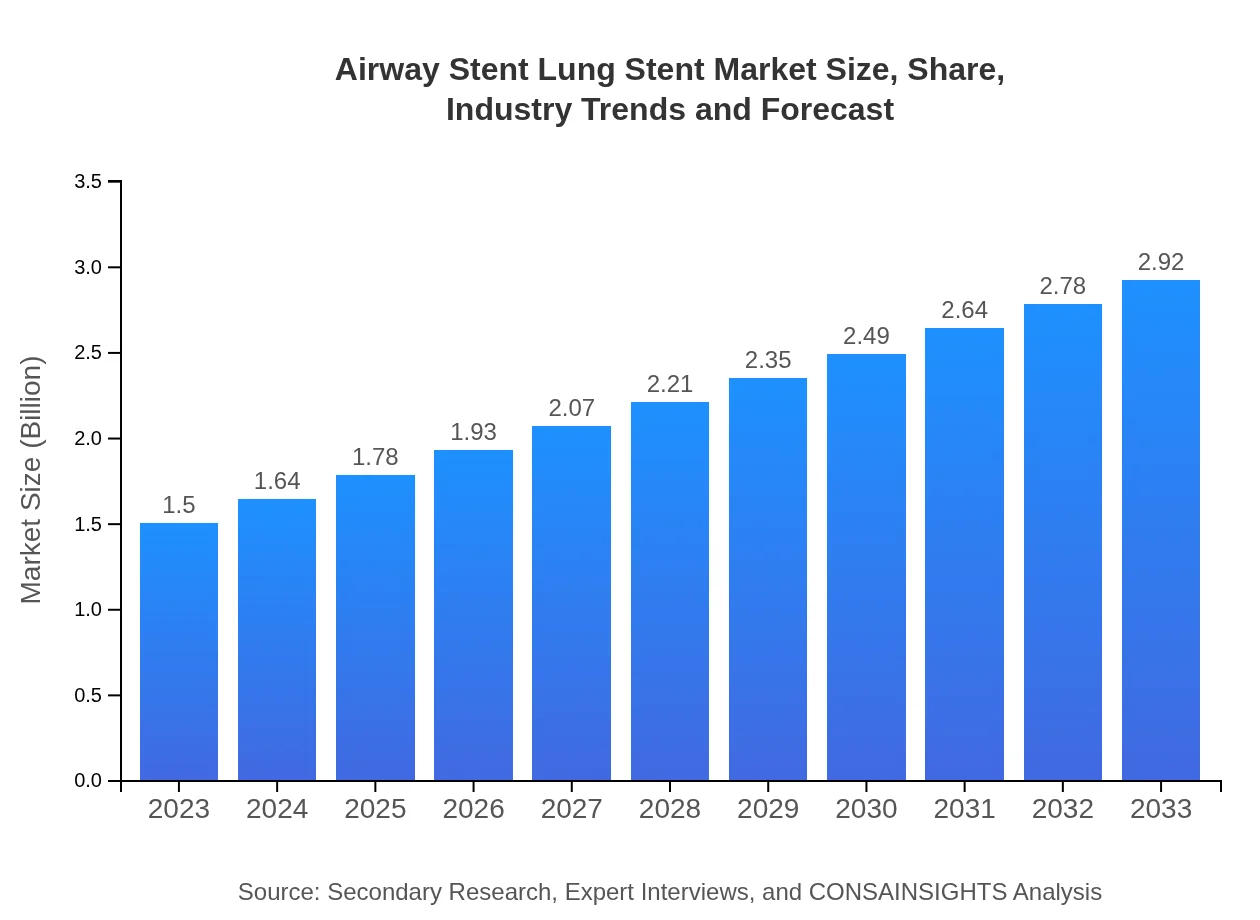
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $2.92 Billion |
| Top Companies | Boston Scientific Corporation, Medtronic plc, Cook Medical, Bard Peripheral Vascular Inc. |
| Last Modified Date | 31 January 2026 |
Airway Stent Lung Stent Market Overview
Customize Airway Stent Lung Stent Market Report market research report
- ✔ Get in-depth analysis of Airway Stent Lung Stent market size, growth, and forecasts.
- ✔ Understand Airway Stent Lung Stent's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Airway Stent Lung Stent
What is the Market Size & CAGR of Airway Stent Lung Stent market in 2023?
Airway Stent Lung Stent Industry Analysis
Airway Stent Lung Stent Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Airway Stent Lung Stent Market Analysis Report by Region
Europe Airway Stent Lung Stent Market Report:
The European market is estimated to grow from $0.36 billion in 2023 to $0.71 billion by 2033, fueled by a growing aging population and initiatives by key players to innovate stent technologies tailored to patients' specific needs.Asia Pacific Airway Stent Lung Stent Market Report:
The Asia Pacific region is projected to experience robust growth, with the market size expected to reach $0.59 billion by 2033 from $0.30 billion in 2023. This growth is driven by increasing healthcare expenditures, rising awareness of advanced treatment modalities, and a growing elderly population susceptible to respiratory diseases.North America Airway Stent Lung Stent Market Report:
North America dominates the market, with a projected increase from $0.51 billion in 2023 to $1.00 billion by 2033. Factors such as advanced healthcare facilities, high prevalence of COPD, and substantial investment in healthcare technology support this growth.South America Airway Stent Lung Stent Market Report:
In South America, the Airway Stent market is anticipated to grow from $0.12 billion in 2023 to $0.23 billion by 2033. The growth is attributed to improving healthcare infrastructure and rising incidences of lung-related diseases, creating an urgent demand for effective treatment solutions.Middle East & Africa Airway Stent Lung Stent Market Report:
In the Middle East and Africa, the market is expected to rise from $0.20 billion in 2023 to $0.40 billion by 2033. This growth can be linked to increasing healthcare investments and rising awareness of respiratory ailments.Tell us your focus area and get a customized research report.
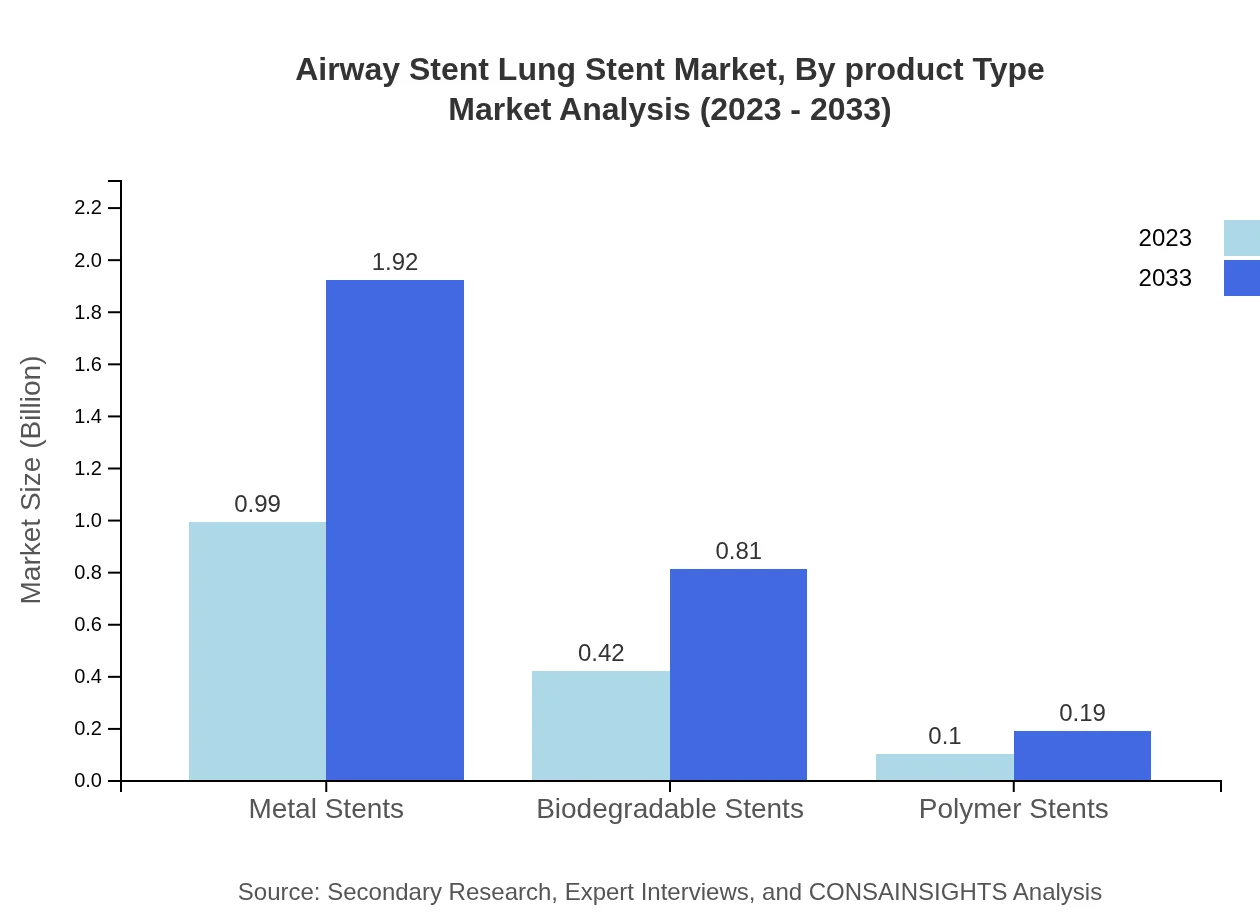
Airway Stent Lung Stent Market Analysis By Product Type
This segment highlights the performance of product types including metal stents, biodegradable stents, and polymer stents. Metal stents hold a market share of 65.78%, driven by their durability and effectiveness. Biodegradable stents, capturing 27.79% of the share, are gaining traction due to their reduced risk of complications. Polymer stents, while smaller at 6.43%, are recognized for their flexibility.
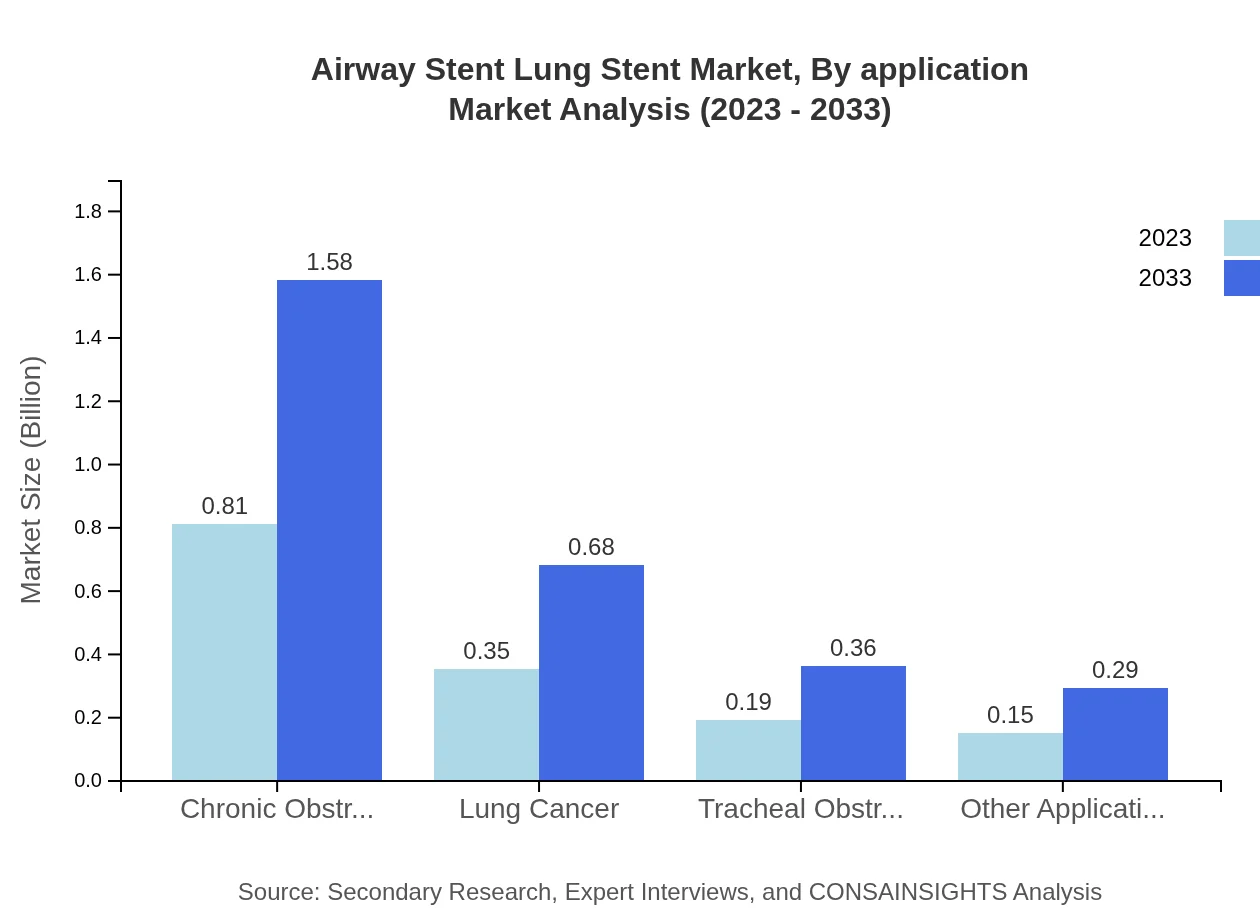
Airway Stent Lung Stent Market Analysis By Application
Key applications include Chronic Obstructive Pulmonary Disease (COPD), lung cancer, and tracheal obstruction. COPD leads the market with a share of 54.14%, reflecting its high prevalence. Lung cancer applications account for 23.31%, followed by tracheal obstruction at 12.45%. This segmentation is critical for directing healthcare resources efficiently.
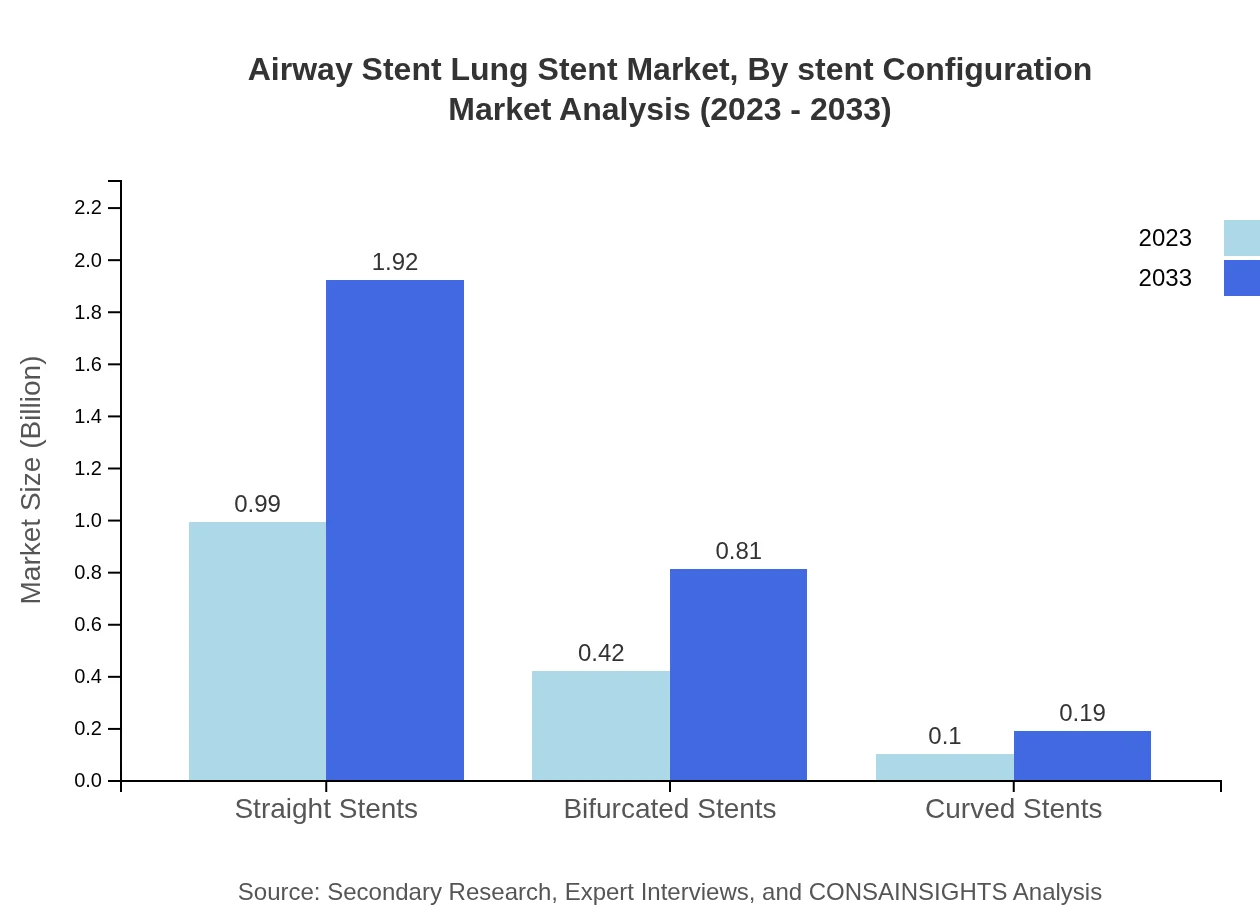
Airway Stent Lung Stent Market Analysis By Stent Configuration
The stent configuration market is divided into straight, bifurcated, and curved stents. Straight stents dominate the market at 65.78%, reflecting their broad usability and effectiveness in various conditions. Bifurcated stents also show substantial interest at 27.79%, while curved stents serve niche applications.
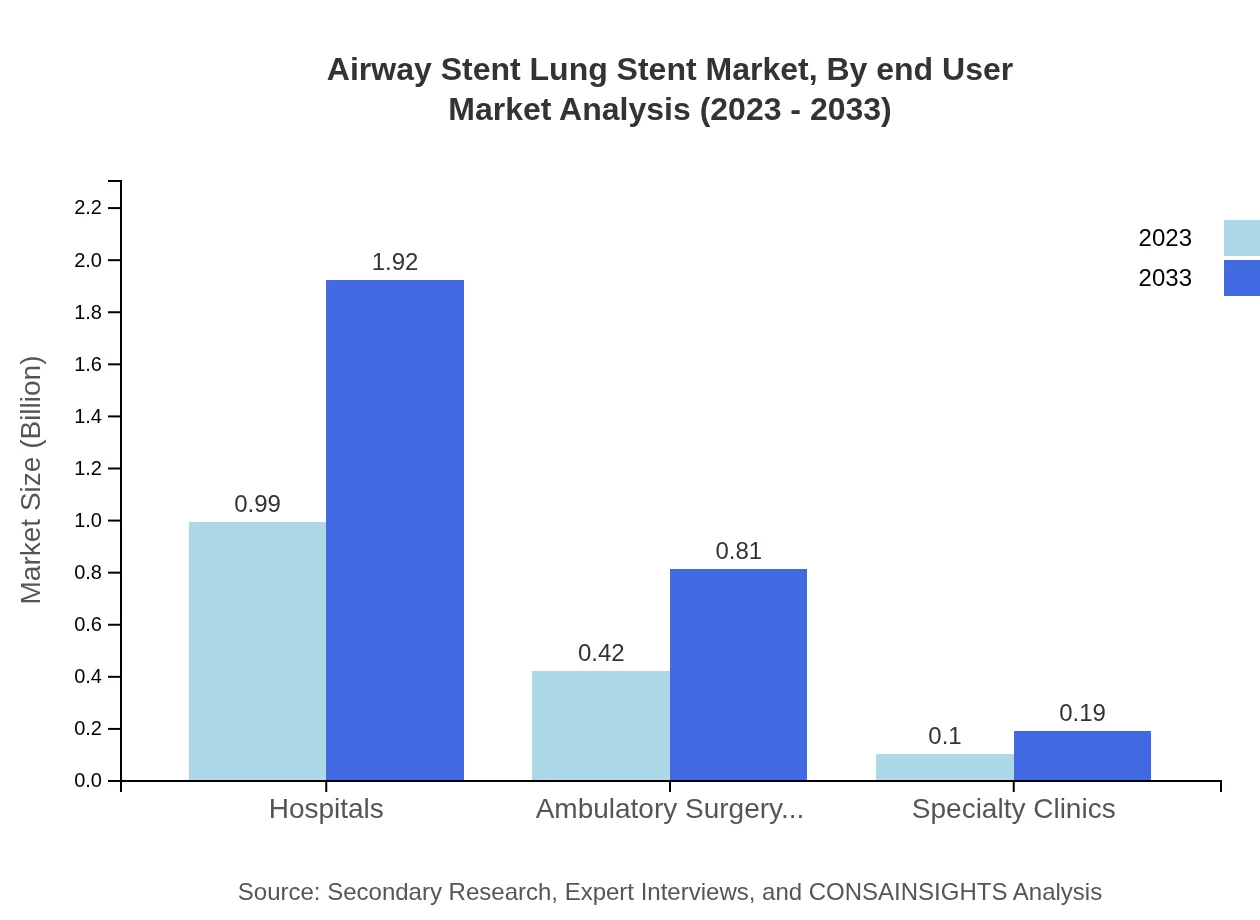
Airway Stent Lung Stent Market Analysis By End User
Hospitals remain the primary end-user, holding a commanding market share of 65.78%. Ambulatory surgery centers and specialty clinics account for 27.79% and 6.43% of the market share, respectively, reflecting an ongoing shift towards outpatient care and minimally invasive procedures.
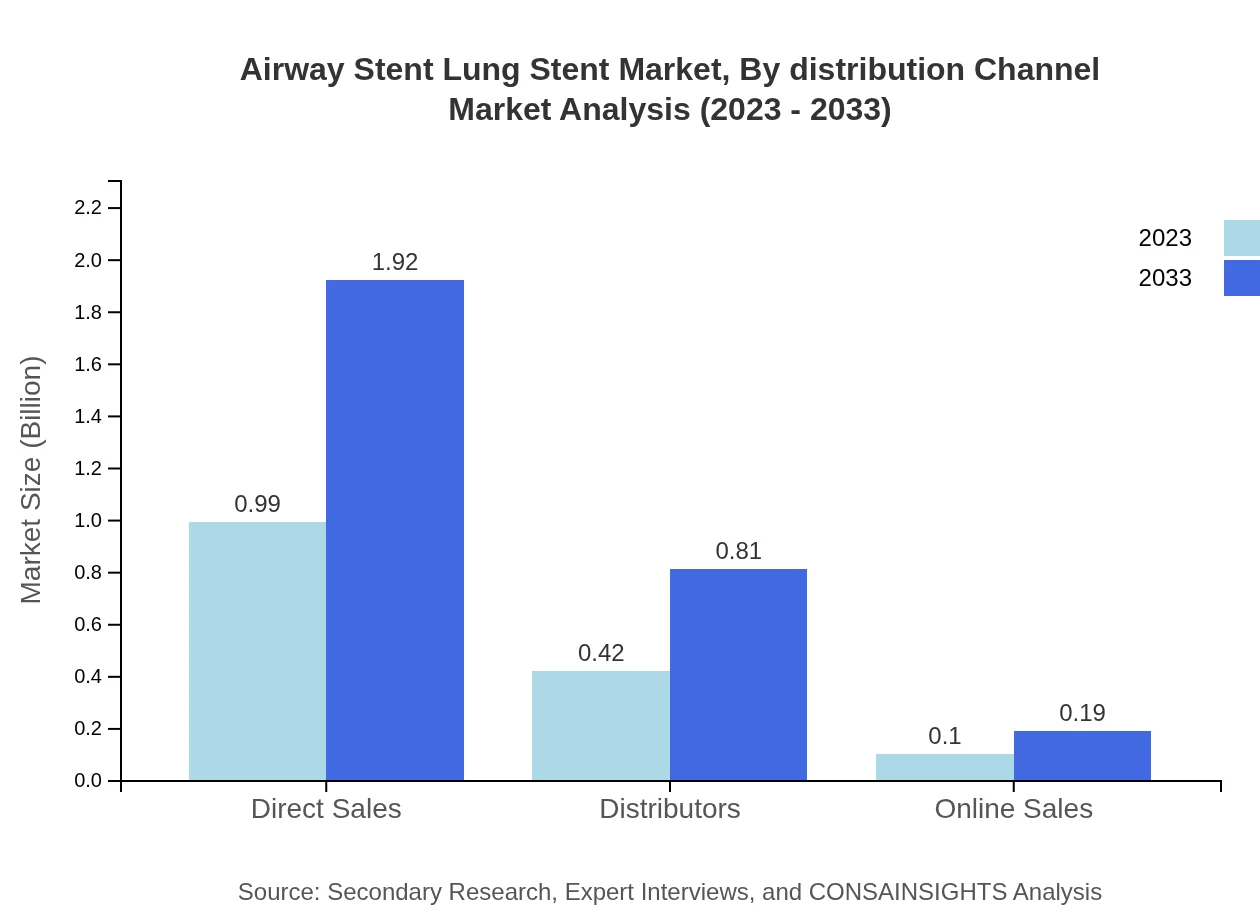
Airway Stent Lung Stent Market Analysis By Distribution Channel
Direct sales lead the distribution channels, capturing 65.78% of the market share, followed by distributors at 27.79% and online sales comprising 6.43%. This trend signifies a strong preference for direct connections between manufacturers and healthcare providers.
Airway Stent Lung Stent Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Airway Stent Lung Stent Industry
Boston Scientific Corporation:
A leading global supplier of innovative medical solutions, Boston Scientific specializes in technologies for minimally invasive interventions across several medical fields, including respiratory health.Medtronic plc:
Medtronic is a major player focused on pioneering medical technology solutions, with significant contributions to airway management through innovative stent designs.Cook Medical:
Cook Medical is known for its diverse range of medical products and devices, including airway stents, catering specifically for challenging clinical conditions.Bard Peripheral Vascular Inc.:
Bard specializes in various vascular products, including stents and filters, focusing on improving patient care and optimizing clinical outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of airway Stent Lung Stent?
The airway stent lung stent market is projected to grow from $1.5 billion in 2023 to substantial figures by 2033, at a compound annual growth rate (CAGR) of 6.7%. This growth reflects a rising demand for advanced respiratory interventions.
What are the key market players or companies in this airway Stent Lung Stent industry?
Key players in the airway stent lung stent market include leading medical device manufacturers who specialize in respiratory health solutions. These companies invest heavily in innovation to develop advanced stenting technologies that cater to an expanding patient population.
What are the primary factors driving the growth in the airway Stent Lung Stent industry?
The growth of the airway stent lung stent market is driven by an increasing incidence of respiratory diseases, advancements in stent technology, and a growing geriatric population. Additionally, rising healthcare expenditure and improved healthcare infrastructure contribute significantly to market expansion.
Which region is the fastest Growing in the airway Stent Lung Stent?
The North America region is expected to be the fastest-growing in the airway stent lung stent market, projected to grow from $0.51 billion in 2023 to $1.00 billion by 2033. This growth is fueled by technological advancements in medical devices and increasing healthcare investments.
Does ConsaInsights provide customized market report data for the airway Stent Lung Stent industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the airway stent lung stent industry. Clients can request specialized analyses that focus on particular regions, demographics, or market segments.
What deliverables can I expect from this airway Stent Lung Stent market research project?
Deliverables for the airway stent lung stent market research project typically include comprehensive market analysis reports, detailed forecasts for market growth, competitive landscape assessments, and insights into market trends and key players.
What are the market trends of airway Stent Lung Stent?
Current trends in the airway stent lung stent market include the shift towards minimally invasive procedures, increased focus on biodegradable stents, and the integration of digital technologies in patient management systems to enhance outcomes.