Alexipharmic Drugs Market Report
Published Date: 31 January 2026 | Report Code: alexipharmic-drugs
Alexipharmic Drugs Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive report on Alexipharmic Drugs provides insights into market size, trends, and forecasts from 2023 to 2033. It covers segmentation, regional analysis, technology impacts, and key industry players, offering a well-rounded perspective for stakeholders and investors.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
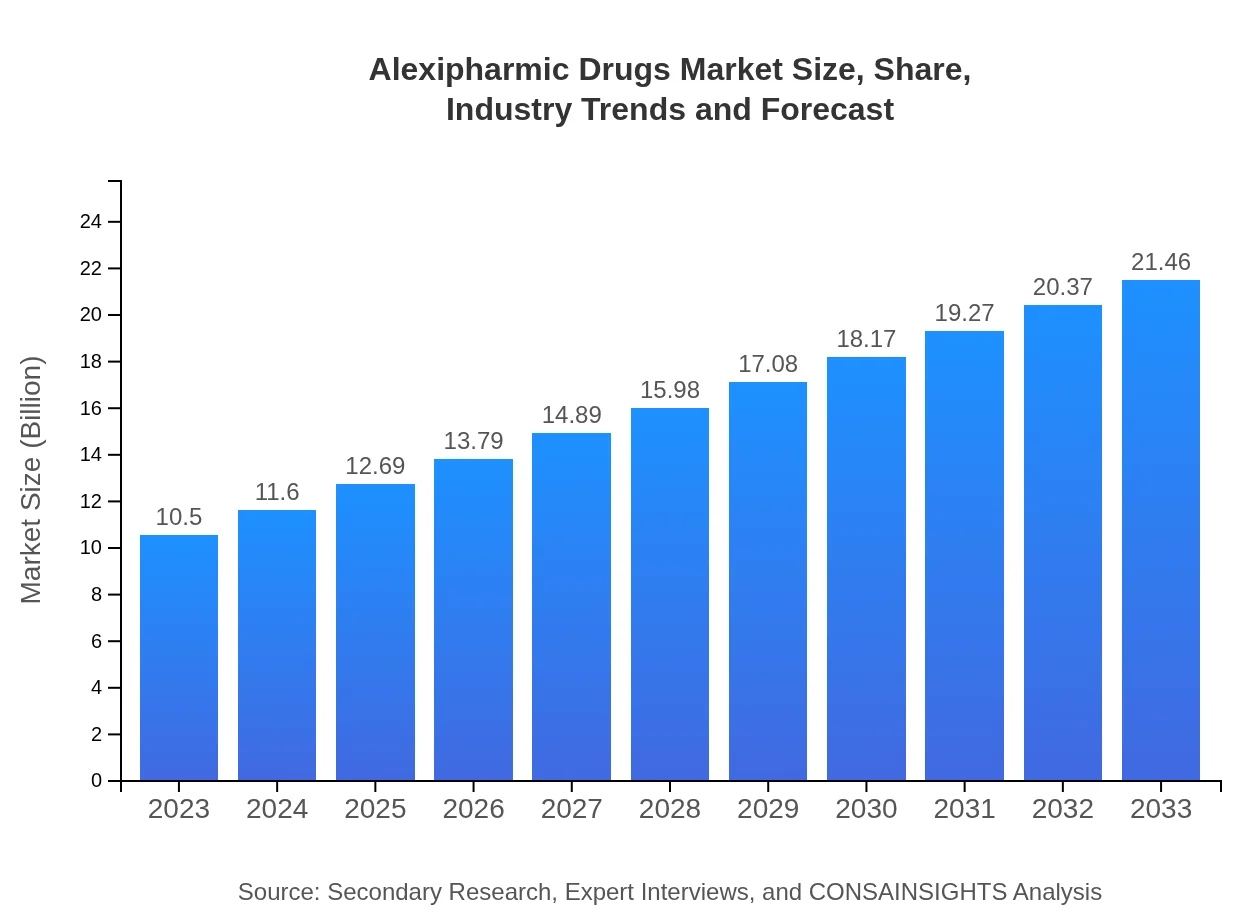
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $21.46 Billion |
| Top Companies | Merck & Co., Bristol-Myers Squibb, GlaxoSmithKline, Bayer AG |
| Last Modified Date | 31 January 2026 |
Alexipharmic Drugs Market Overview
Customize Alexipharmic Drugs Market Report market research report
- ✔ Get in-depth analysis of Alexipharmic Drugs market size, growth, and forecasts.
- ✔ Understand Alexipharmic Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Alexipharmic Drugs
What is the Market Size & CAGR of Alexipharmic Drugs market in 2023?
Alexipharmic Drugs Industry Analysis
Alexipharmic Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Alexipharmic Drugs Market Analysis Report by Region
Europe Alexipharmic Drugs Market Report:
In Europe, the market size is expected to increase from $3.44 billion in 2023 to $7.03 billion by 2033. This growth is backed by stringent health regulations and a robust pharmaceutical sector focused on antidote development.Asia Pacific Alexipharmic Drugs Market Report:
In the Asia Pacific, the market is expected to grow from $1.65 billion in 2023 to $3.38 billion by 2033. Growth in this region can be attributed to the increasing prevalence of toxic exposure and improvements in healthcare infrastructure, combined with rising awareness regarding Alexipharmic Drugs.North America Alexipharmic Drugs Market Report:
North America is anticipated to expand from $3.86 billion in 2023 to $7.89 billion by 2033. This region is driven by advanced healthcare systems, government investments in research, and the prevalence of substance abuse, which increases the demand for antidotes like Naloxone.South America Alexipharmic Drugs Market Report:
The South American market is projected to grow from $0.66 billion in 2023 to $1.34 billion by 2033. Factors such as improving healthcare access and referral systems are contributing to the growth of antidote use in emergency situations.Middle East & Africa Alexipharmic Drugs Market Report:
The Middle East and Africa market will rise from $0.89 billion in 2023 to $1.82 billion by 2033, driven by increased awareness of poison management and the establishment of better healthcare facilities.Tell us your focus area and get a customized research report.
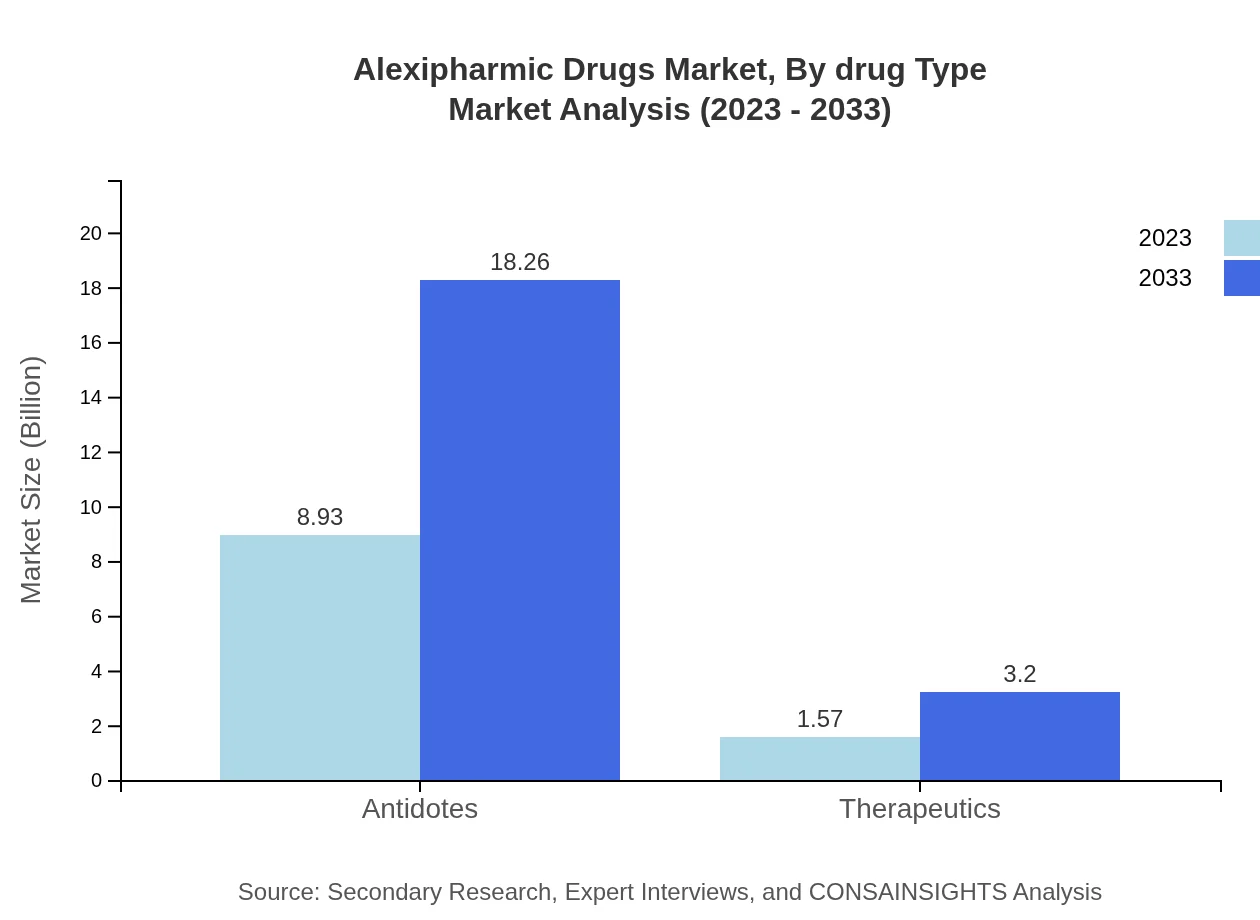
Alexipharmic Drugs Market Analysis By Drug Type
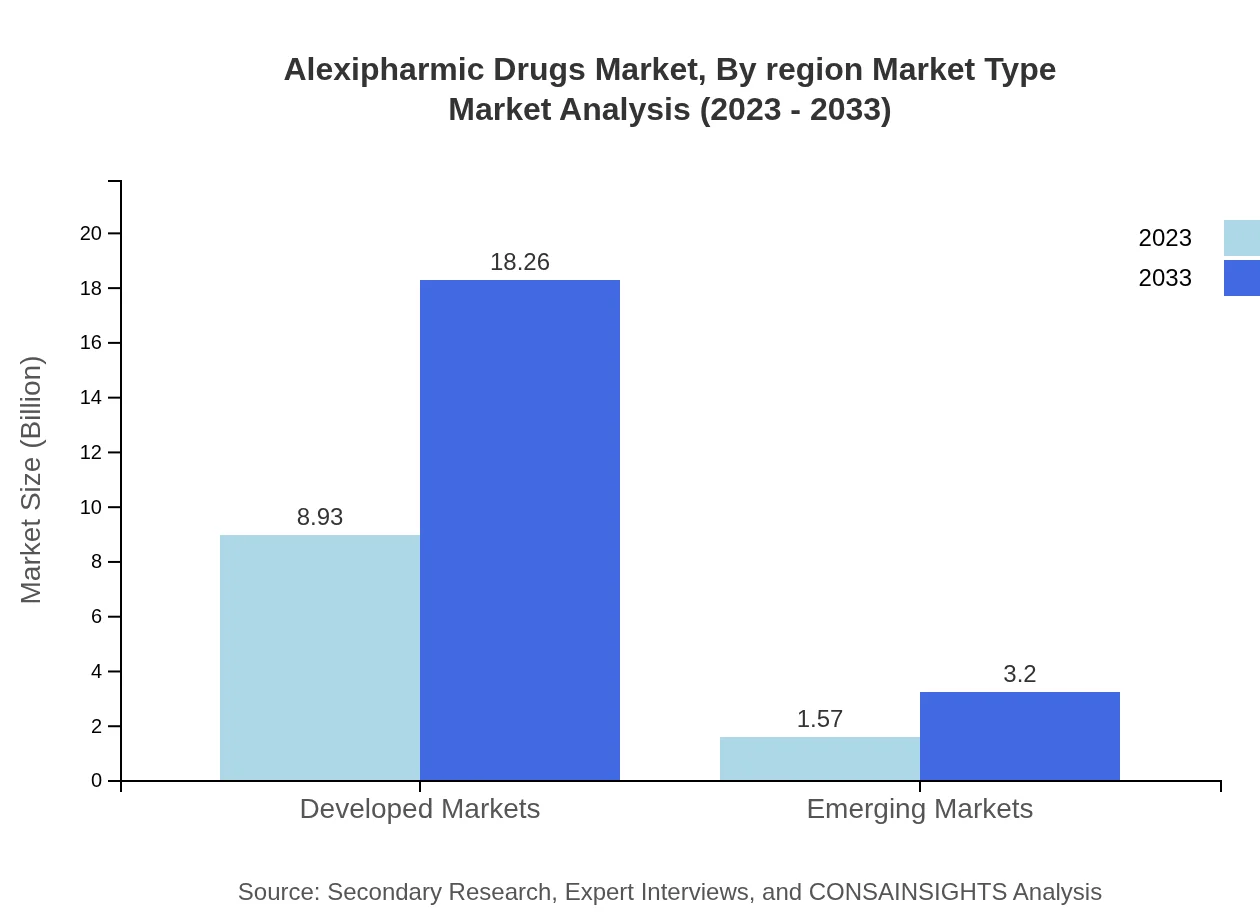
Antidotes are the leading segment, with a market value projected to grow to $18.26 billion by 2033 from $8.93 billion in 2023, holding 85.09% market share throughout this period. In contrast, therapeutics, while smaller in size, are gaining traction with growth expected from $1.57 billion to $3.20 billion, accounting for 14.91% share.
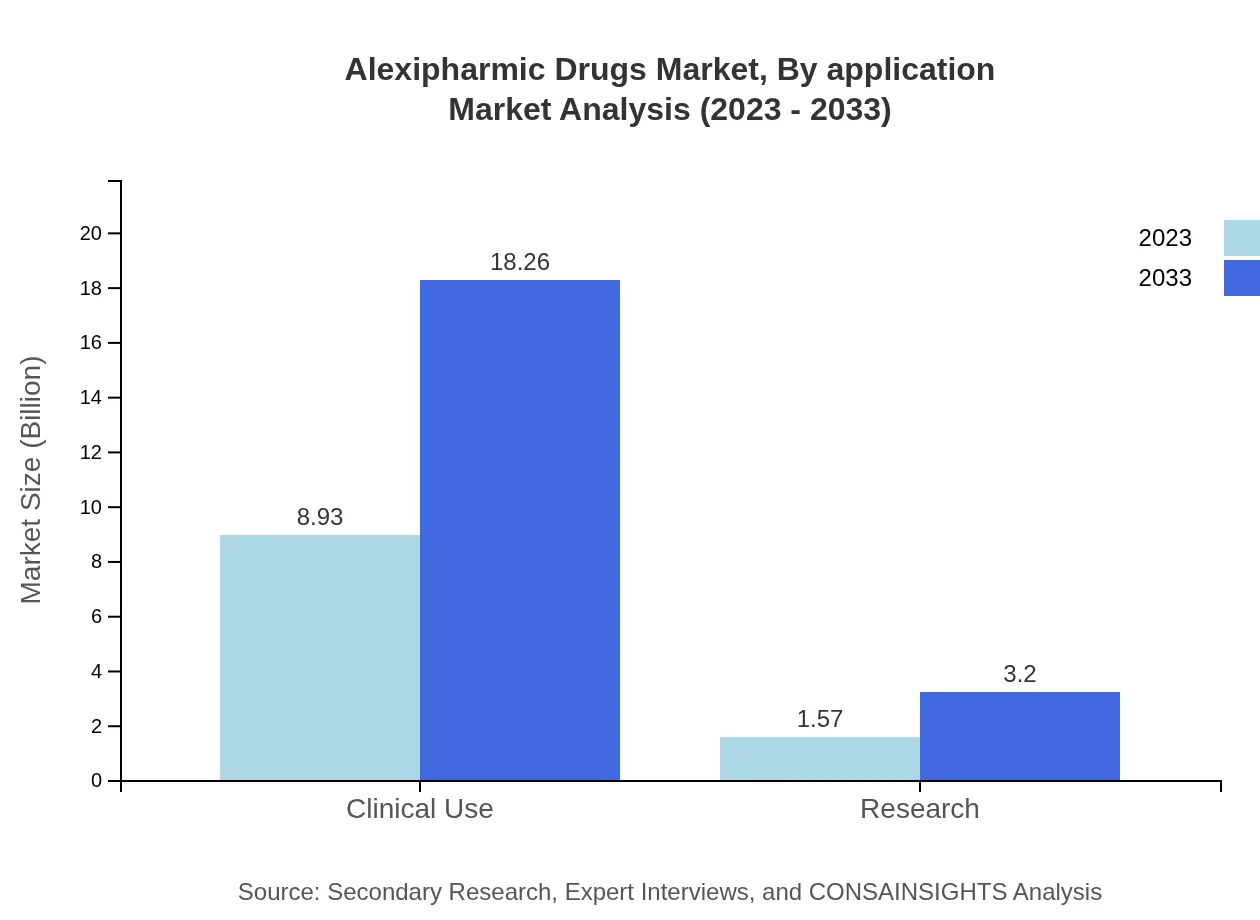
Alexipharmic Drugs Market Analysis By Application
Similar to the drug type segmentation, clinical use remains the key focus, projected to grow from $8.93 billion to $18.26 billion, while research applications are anticipated to grow from $1.57 billion to $3.20 billion. Clinical and research uses globally maintain equivalent importance in addressing health emergencies and discovering new antidotes.
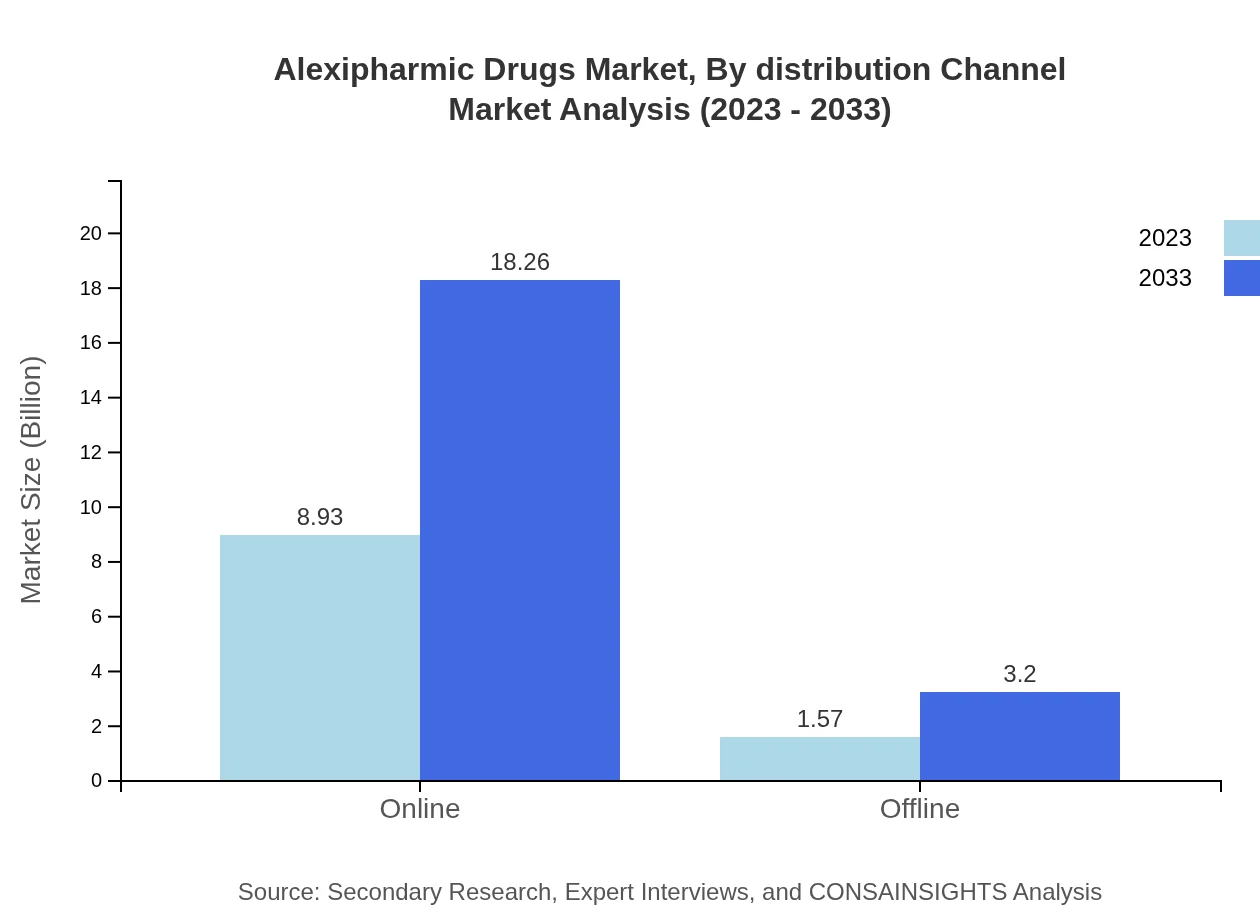
Alexipharmic Drugs Market Analysis By Distribution Channel
The online distribution channel for Alexipharmic Drugs is set to witness significant growth, rising from $8.93 billion in 2023 to $18.26 billion by 2033. Offline channels, though currently accounting for a smaller market at $1.57 billion, are also projected to grow to $3.20 billion over the same period, representing the essential role of pharmacies in drug distribution.
Alexipharmic Drugs Market Analysis By Region Market Type
This segment includes all regions mentioned earlier, highlighting the path to market maturity for antidotes in areas like North America and Europe while emphasizing the rapid growth in Asia Pacific and Latin America.
Alexipharmic Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Alexipharmic Drugs Industry
Merck & Co.:
Merck is a leading pharmaceutical company known for its extensive range of antidotes and therapeutics, heavily invested in research to develop novel medications.Bristol-Myers Squibb:
This global biopharmaceutical company focuses on providing advanced medicines, contributing significantly to the antidote market with innovative research and development.GlaxoSmithKline:
GSK is committed to developing a diversified portfolio of healthcare solutions. Their antidotes address a wide range of poisoning scenarios.Bayer AG:
Bayer is recognized for its contributions to the pharmaceutical industry, focusing on comprehensive antidote solutions for various toxic exposures.We're grateful to work with incredible clients.









FAQs
What is the market size of alexipharmic Drugs?
The global market size for alexipharmic drugs is valued at approximately $10.5 billion in 2023 with an expected CAGR of 7.2% over the next decade, indicating robust growth driven by increasing demand for antidotes and therapeutics.
What are the key market players or companies in the alexipharmic Drugs industry?
Key players in the alexipharmic drugs market include major pharmaceutical companies known for antidote development, therapeutic solutions, and research applications. A focus on innovation and strategic partnerships enhances their market presence and competitive edge.
What are the primary factors driving the growth in the alexipharmic Drugs industry?
Growth in the alexipharmic drugs market is driven by increasing prevalence of poisoning cases, rising awareness of available antidotes, and advancements in medical treatments, alongside a growing demand for innovative therapeutic solutions.
Which region is the fastest Growing in the alexipharmic Drugs?
The fastest-growing region for alexipharmic drugs is Europe, projected to grow from $3.44 billion in 2023 to $7.03 billion by 2033, driven by advancements in healthcare and increasing investment in pharmaceutical research.
Does ConsaInsights provide customized market report data for the alexipharmic Drugs industry?
Yes, ConsaInsights provides customized market report data for the alexipharmic drugs industry, allowing clients to obtain tailored insights that meet their specific research objectives and business needs.
What deliverables can I expect from this alexipharmic Drugs market research project?
Deliverables from the alexipharmic drugs market research project include comprehensive market analysis reports, regional and segment data, competitive landscape insights, and future growth forecasts, equipping clients with actionable intelligence.
What are the market trends of alexipharmic Drugs?
Current trends in the alexipharmic drugs market include a shift towards online distribution, increased investment in research and development for antidotes, and a focus on expanding therapeutic applications to enhance patient outcomes.