Alzheimer S Disease Diagnostics And Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: alzheimer-s-disease-diagnostics-and-therapeutics
Alzheimer S Disease Diagnostics And Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This market report provides an in-depth analysis of the Alzheimer’s Disease Diagnostics and Therapeutics sector, covering market trends, forecasts (2023-2033), and regional insights. It highlights current challenges, growth opportunities, and key players for stakeholders to consider.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
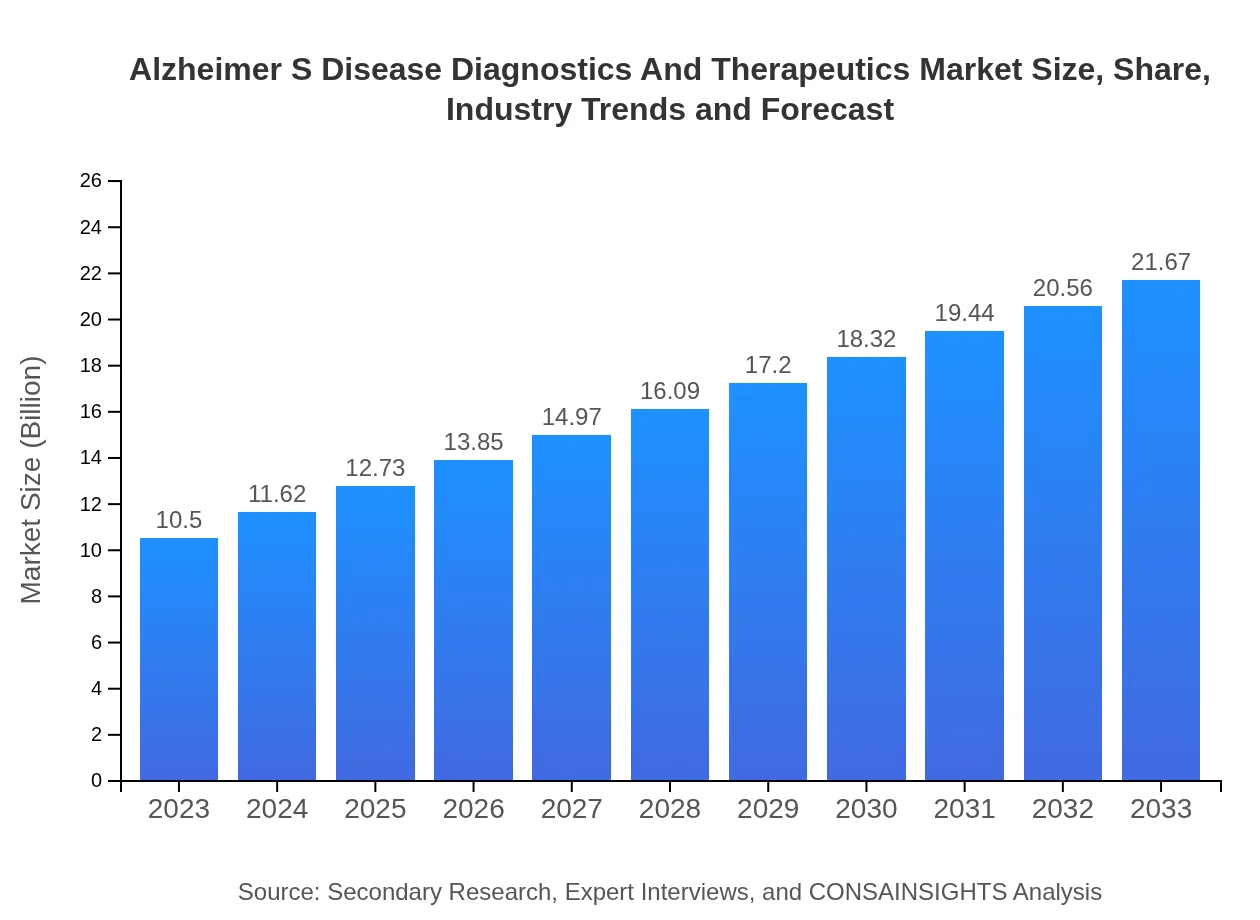
| 2023 Market Size | $10.50 Billion |
| CAGR (2023-2033) | 7.3% |
| 2033 Market Size | $21.67 Billion |
| Top Companies | Roche Diagnostics, Eli Lilly, Biogen, Novartis |
| Last Modified Date | 31 January 2026 |
Alzheimer S Disease Diagnostics And Therapeutics Market Overview
Customize Alzheimer S Disease Diagnostics And Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Alzheimer S Disease Diagnostics And Therapeutics market size, growth, and forecasts.
- ✔ Understand Alzheimer S Disease Diagnostics And Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Alzheimer S Disease Diagnostics And Therapeutics
What is the Market Size & CAGR of Alzheimer S Disease Diagnostics And Therapeutics market in 2023?
Alzheimer S Disease Diagnostics And Therapeutics Industry Analysis
Alzheimer S Disease Diagnostics And Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Alzheimer S Disease Diagnostics And Therapeutics Market Analysis Report by Region
Europe Alzheimer S Disease Diagnostics And Therapeutics Market Report:
In Europe, the market size is estimated at $3.20 billion in 2023, reaching $6.60 billion by 2033. Supportive healthcare policies, coupled with a focus on enhancing the quality of Alzheimer’s care, foster growth in this region.Asia Pacific Alzheimer S Disease Diagnostics And Therapeutics Market Report:
In the Asia-Pacific region, the Alzheimer's Disease Diagnostics and Therapeutics market is projected to grow from $1.83 billion in 2023 to $3.77 billion in 2033. This growth is driven by rising elderly populations and increased awareness of cognitive health, alongside governmental initiatives to support research and health innovations.North America Alzheimer S Disease Diagnostics And Therapeutics Market Report:
North America leads the market with a valuation of $4.00 billion in 2023, anticipated to grow to $8.25 billion by 2033. The region benefits from advanced healthcare infrastructures, substantial R&D investments, and high healthcare expenditure, driving innovations in diagnostics and therapeutics.South America Alzheimer S Disease Diagnostics And Therapeutics Market Report:
The South American market is relatively smaller, valued at $0.18 billion in 2023 and expected to reach $0.36 billion by 2033. This region faces healthcare access challenges, but increasing research investments and regional collaborations are aiding market growth.Middle East & Africa Alzheimer S Disease Diagnostics And Therapeutics Market Report:
The Middle East and Africa market is valued at $1.30 billion in 2023 and is projected to grow to $2.69 billion by 2033. Growing urbanization and economic development are increasing healthcare access, although the market still faces challenges regarding awareness and infrastructure.Tell us your focus area and get a customized research report.
Alzheimer S Disease Diagnostics And Therapeutics Market Analysis By Diagnostic Method
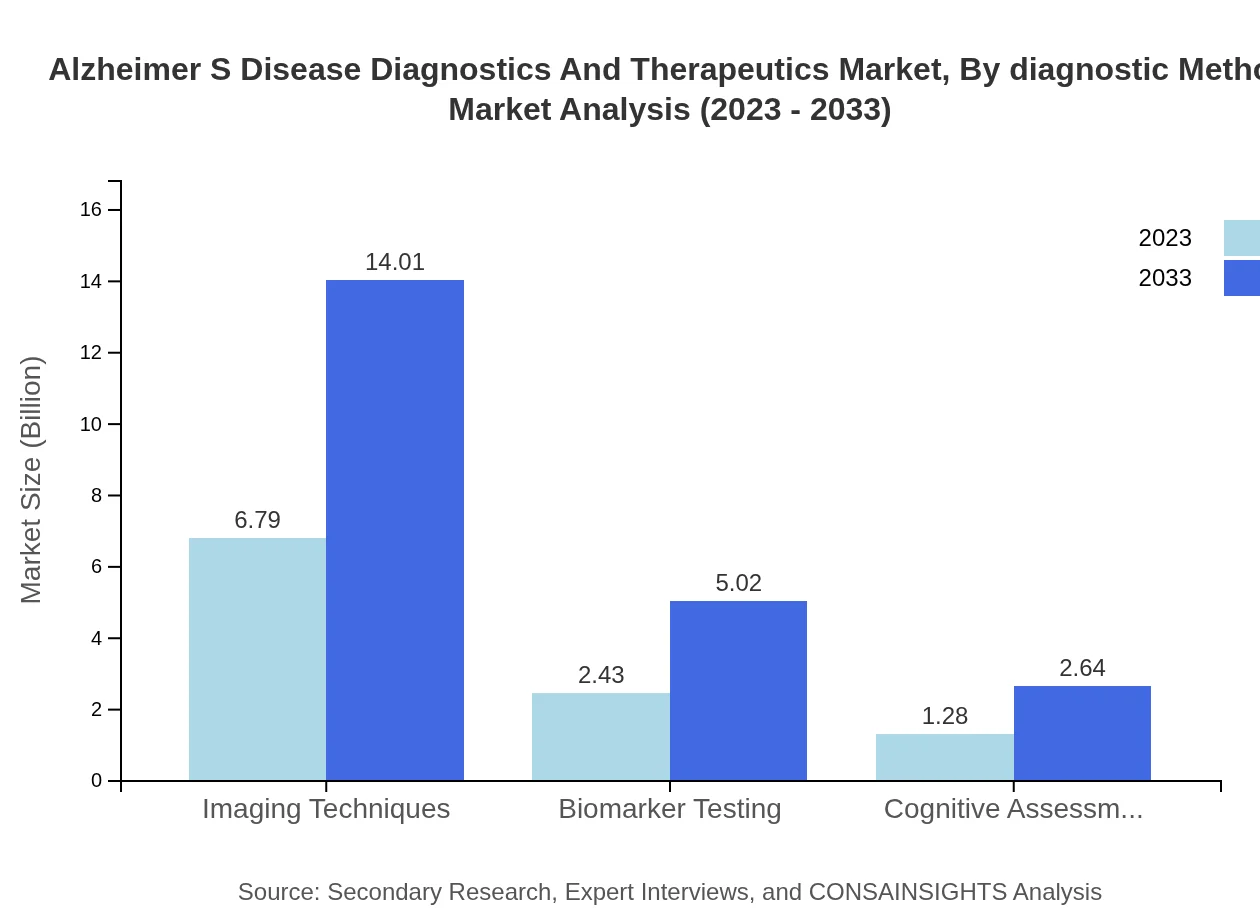
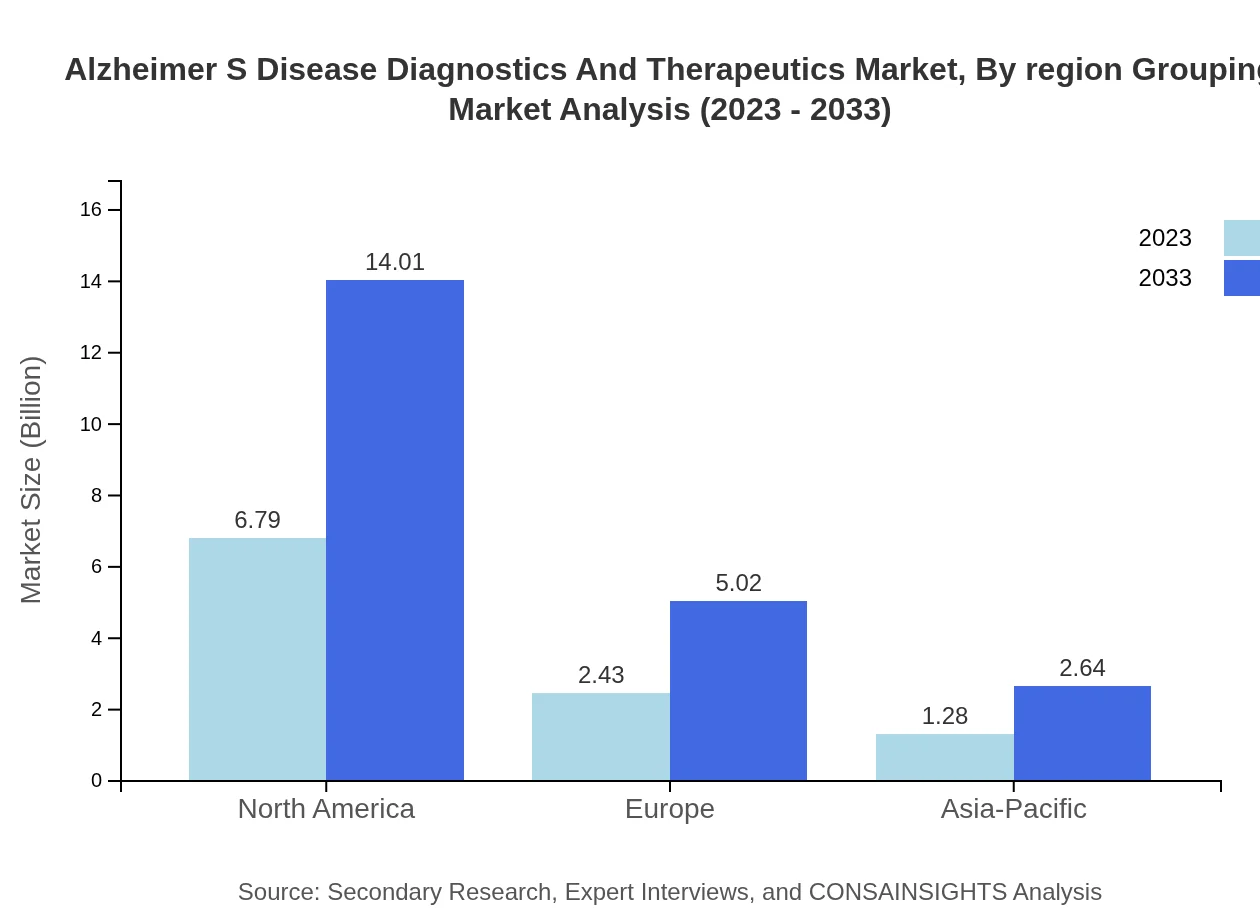
The diagnostics segment focuses on imaging techniques, biomarker testing, and cognitive assessments. In 2023, the imaging techniques market is valued at $6.79 billion with expectations to reach $14.01 billion by 2033. Biomarker testing holds a market share of $2.43 billion and is expected to increase to $5.02 billion, while cognitive assessments' market size is $1.28 billion, growing to $2.64 billion by 2033.
Alzheimer S Disease Diagnostics And Therapeutics Market Analysis By Therapeutic Approach
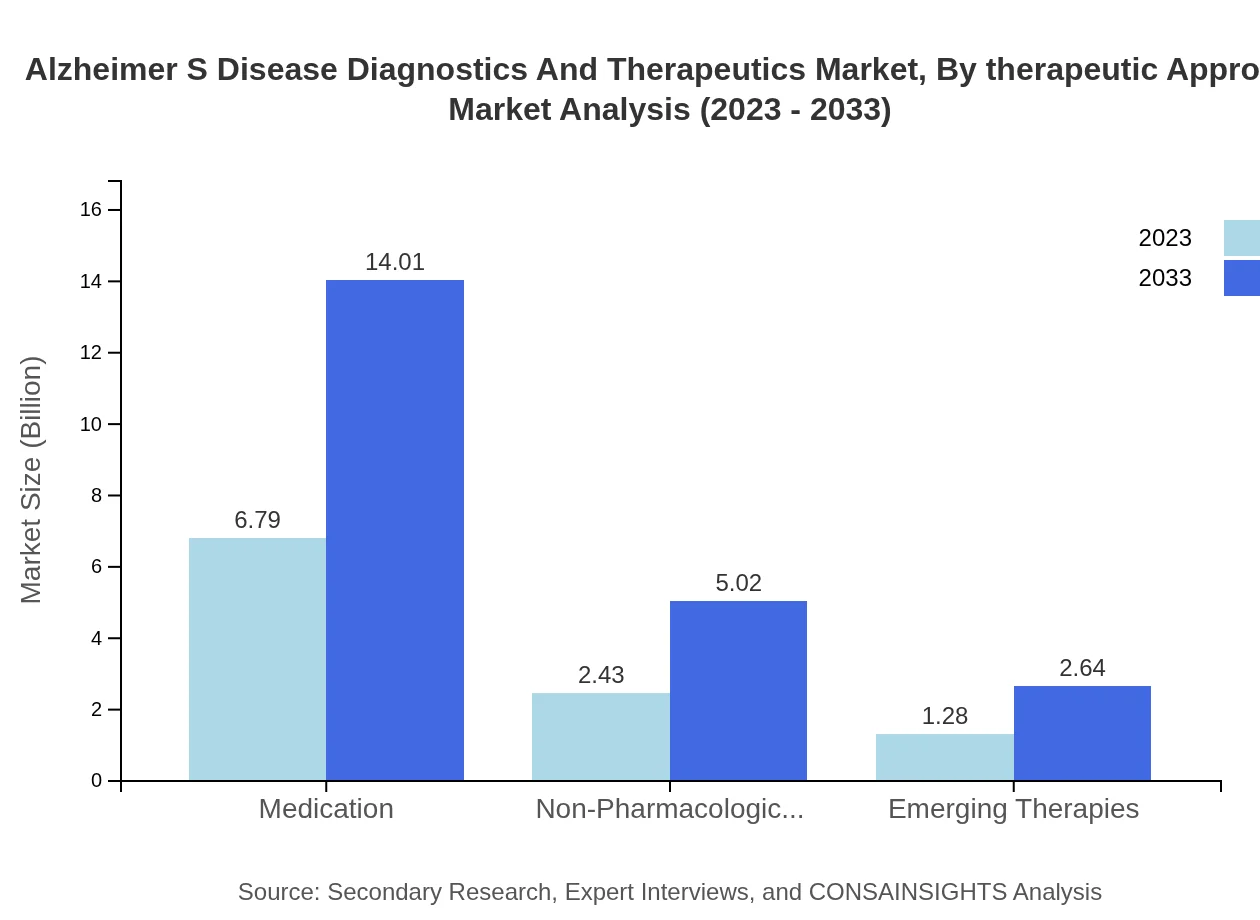
The therapeutic approach segment includes medication and non-pharmacological interventions. The medication market stands tall at $6.79 billion in 2023, anticipated to reach $14.01 billion by 2033, holding a stable market share. Meanwhile, non-pharmacological interventions have a share of $2.43 billion, expected to grow to $5.02 billion, emphasizing integrative care approaches.
Alzheimer S Disease Diagnostics And Therapeutics Market Analysis By End User
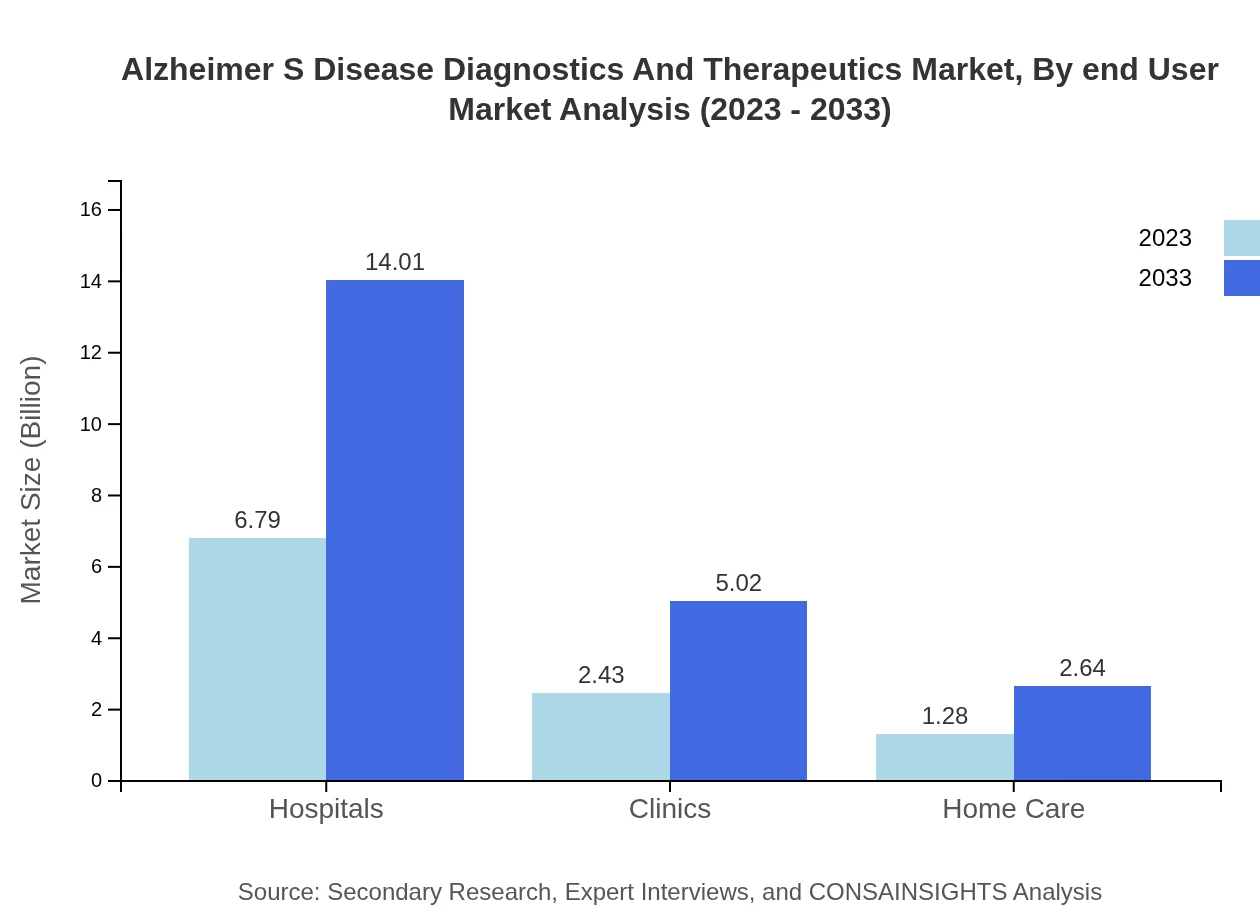
Hospitals dominate the market with a size of $6.79 billion in 2023, maintaining a 64.66% share through to 2033. Clinics contribute significantly with a current market of $2.43 billion, expected to rise to $5.02 billion. Home care settings, valued at $1.28 billion in 2023, are positioned for growth, increasing concern for Alzheimer’s patient care within the home environment.
Alzheimer S Disease Diagnostics And Therapeutics Market Analysis By Region Grouping
Each region is experiencing unique growth patterns, with North America leading due to robust healthcare policies and funding. Europe follows closely, with notable growth, especially in diagnostics. The Asia-Pacific and Middle East regions are rapidly catching up with increased focus and resource allocation dedicated to Alzheimer’s care.
Alzheimer S Disease Diagnostics And Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Alzheimer S Disease Diagnostics And Therapeutics Industry
Roche Diagnostics:
Roche is a pioneer in the Alzheimer’s diagnostic solutions space, developing advanced biomarker tests and imaging solutions that significantly enhance early diagnostic capabilities.Eli Lilly:
Eli Lilly is known for its extensive research in Alzheimer’s therapeutics, particularly in developing novel medication aimed at altering disease progression.Biogen:
Biogen is a key player in Alzheimer’s therapeutics, recognized for its advancements in monoclonal antibodies and therapies that target amyloid plaques.Novartis:
Novartis invests heavily in research and development of both diagnostics and treatments for Alzheimer’s, emphasizing technological innovation and patient-centric solutions.We're grateful to work with incredible clients.









FAQs
What is the market size of Alzheimer’s disease diagnostics and therapeutics?
The global market size for Alzheimer's disease diagnostics and therapeutics is projected to reach approximately $10.5 billion by 2033, growing at a compound annual growth rate (CAGR) of 7.3% from its current value.
What are the key market players or companies in the Alzheimer’s disease diagnostics and therapeutics industry?
Key players in the Alzheimer's disease diagnostics and therapeutics market include major pharmaceutical companies focusing on innovative treatments, diagnostic tool manufacturers, and research institutions dedicated to advancing Alzheimer's disease management.
What are the primary factors driving the growth in the Alzheimer’s disease diagnostics and therapeutics industry?
Factors driving growth include an aging population, increased awareness of Alzheimer's disease, advancements in diagnostic technologies, and significant research investments leading to novel therapeutics and biomarkers for early detection.
Which region is the fastest Growing in the Alzheimer’s disease diagnostics and therapeutics?
The fastest-growing region for Alzheimer's disease diagnostics and therapeutics is projected to be Europe, with market growth from $3.20 billion in 2023 to $6.60 billion by 2033, demonstrating robust demand and investment.
Does ConsaInsights provide customized market report data for the Alzheimer’s disease diagnostics and therapeutics industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs, enabling clients to gain insights relevant to Alzheimer’s disease diagnostics and therapeutics based on their strategic objectives.
What deliverables can I expect from this Alzheimer’s disease diagnostics and therapeutics market research project?
Deliverables from the market research project include comprehensive market analysis, segmentation data, competitive landscape assessments, growth forecasts, and actionable insights tailored to stakeholders in the Alzheimer’s sector.
What are the market trends of Alzheimer’s disease diagnostics and therapeutics?
Current market trends include increasing investments in research for innovative therapies, a rise in personalized medicine approaches, enhanced focus on early detection tools like biomarker testing, and a shift toward integrative care strategies.