Amniocentesis Needle Market Report
Published Date: 31 January 2026 | Report Code: amniocentesis-needle
Amniocentesis Needle Market Size, Share, Industry Trends and Forecast to 2033
This report provides an extensive analysis of the Amniocentesis Needle market, covering key trends, market sizes, segmentations, and regional insights from 2023 to 2033. It aims to equip stakeholders with essential data for strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
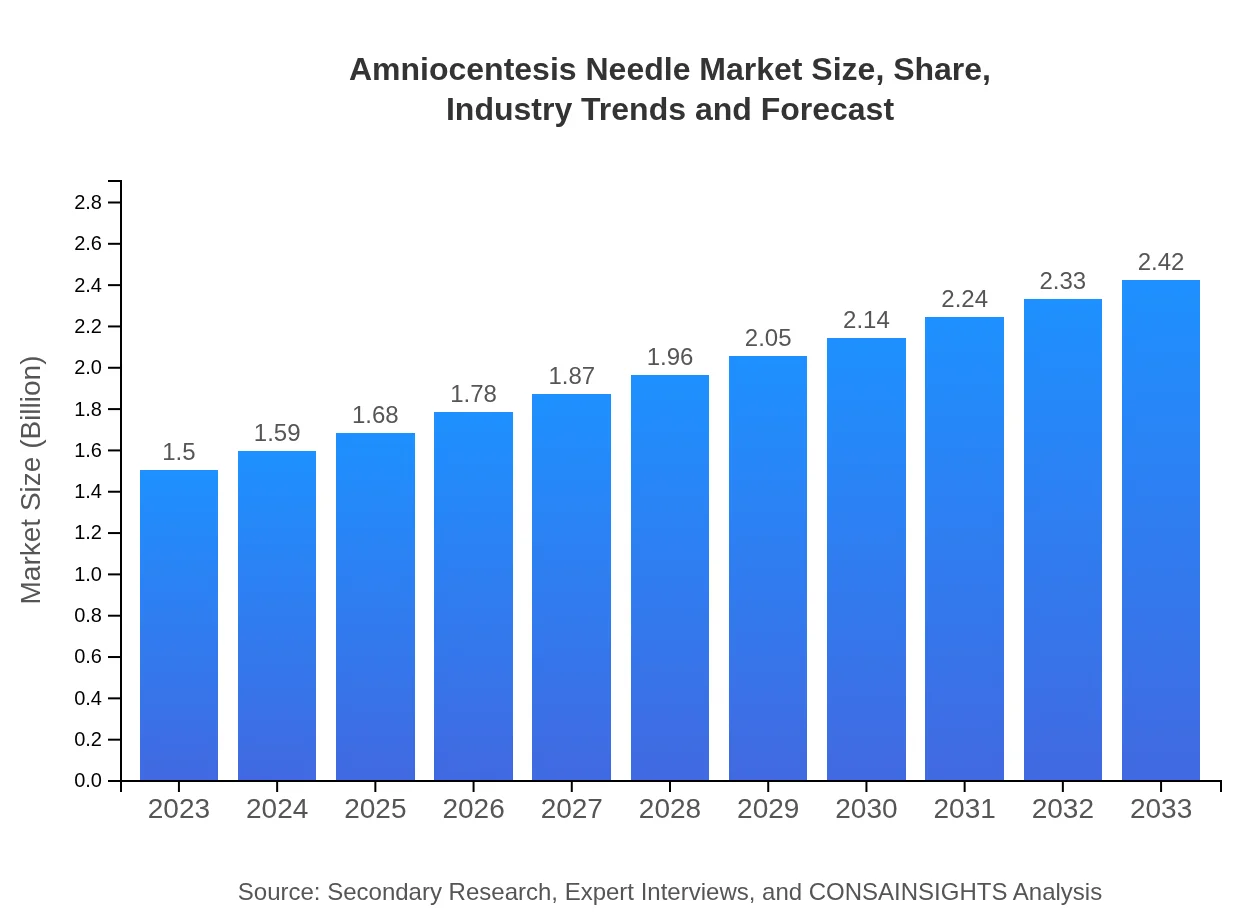
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 4.8% |
| 2033 Market Size | $2.42 Billion |
| Top Companies | Medtronic , Becton, Dickinson and Company (BD), Cook Medical |
| Last Modified Date | 31 January 2026 |
Amniocentesis Needle Market Overview
Customize Amniocentesis Needle Market Report market research report
- ✔ Get in-depth analysis of Amniocentesis Needle market size, growth, and forecasts.
- ✔ Understand Amniocentesis Needle's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Amniocentesis Needle
What is the Market Size & CAGR of Amniocentesis Needle market in 2033?
Amniocentesis Needle Industry Analysis
Amniocentesis Needle Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Amniocentesis Needle Market Analysis Report by Region
Europe Amniocentesis Needle Market Report:
In Europe, the market was valued at $0.44 billion in 2023, with expectations of growing to $0.71 billion by 2033. The European region benefits from stringent health regulations ensuring device safety and efficacy, along with increasing awareness and acceptance of prenatal testing, which drives significant utilization of amniocentesis procedures across member states.Asia Pacific Amniocentesis Needle Market Report:
The Asia Pacific region accounted for a market size of $0.28 billion in 2023, projected to grow to $0.46 billion by 2033. This growth is largely driven by increasing health awareness, improving healthcare infrastructure, and higher birth rates, leading to a demand for prenatal diagnostic procedures. Countries like China and India are witnessing significant investments in maternal healthcare, further supporting this trend.North America Amniocentesis Needle Market Report:
North America is projected to lead the Amniocentesis Needle market with a size of $0.57 billion in 2023, reaching $0.92 billion by 2033. The high prevalence of prenatal disorders, combined with advanced healthcare systems and insurance coverage for prenatal diagnostics, fuels this market growth. The United States, in particular, showcases a robust healthcare infrastructure supporting prenatal testing.South America Amniocentesis Needle Market Report:
In South America, the market is relatively small, with a size of $0.03 billion in 2023, anticipated to rise to $0.06 billion by 2033. Limited access to healthcare and economic challenges hinder rapid growth; however, awareness campaigns and improved accessibility in urban areas are expected to stimulate market expansion.Middle East & Africa Amniocentesis Needle Market Report:
The Middle East and Africa region's market is set to grow from $0.17 billion in 2023 to $0.28 billion by 2033. Factors such as rising healthcare expenditure and initiatives to enhance maternal and child health significantly contribute to this upward trajectory, despite facing infrastructural challenges in several countries.Tell us your focus area and get a customized research report.
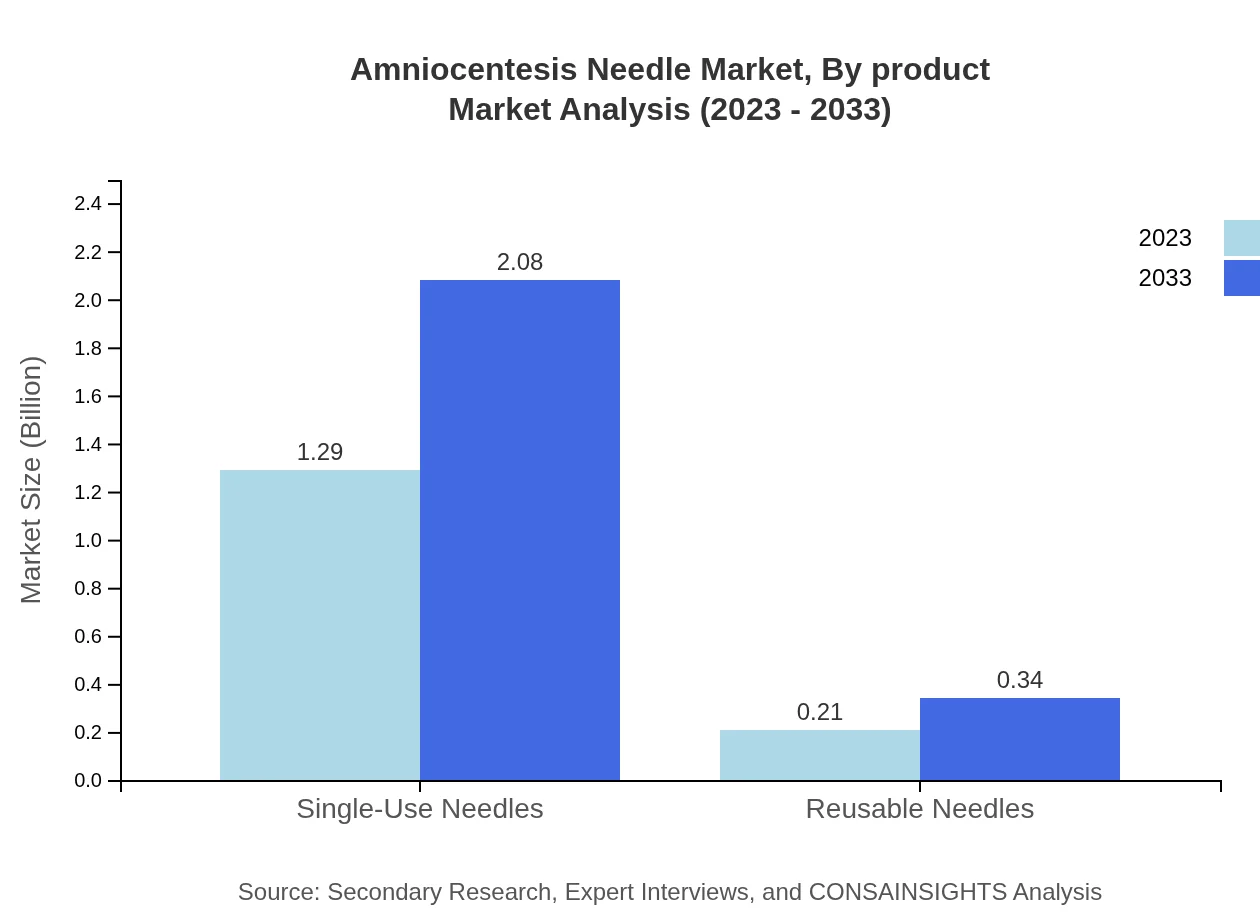
Amniocentesis Needle Market Analysis By Product
The market for Amniocentesis Needles is segmented primarily into single-use and reusable needles. In 2023, single-use needles dominated the market with an estimated size of $1.29 billion, expected to reach $2.08 billion by 2033, holding approximately 85.78% of the market share. Reusable needles are projected to expand from $0.21 billion in 2023 to $0.34 billion in 2033, capturing 14.22% of the share.
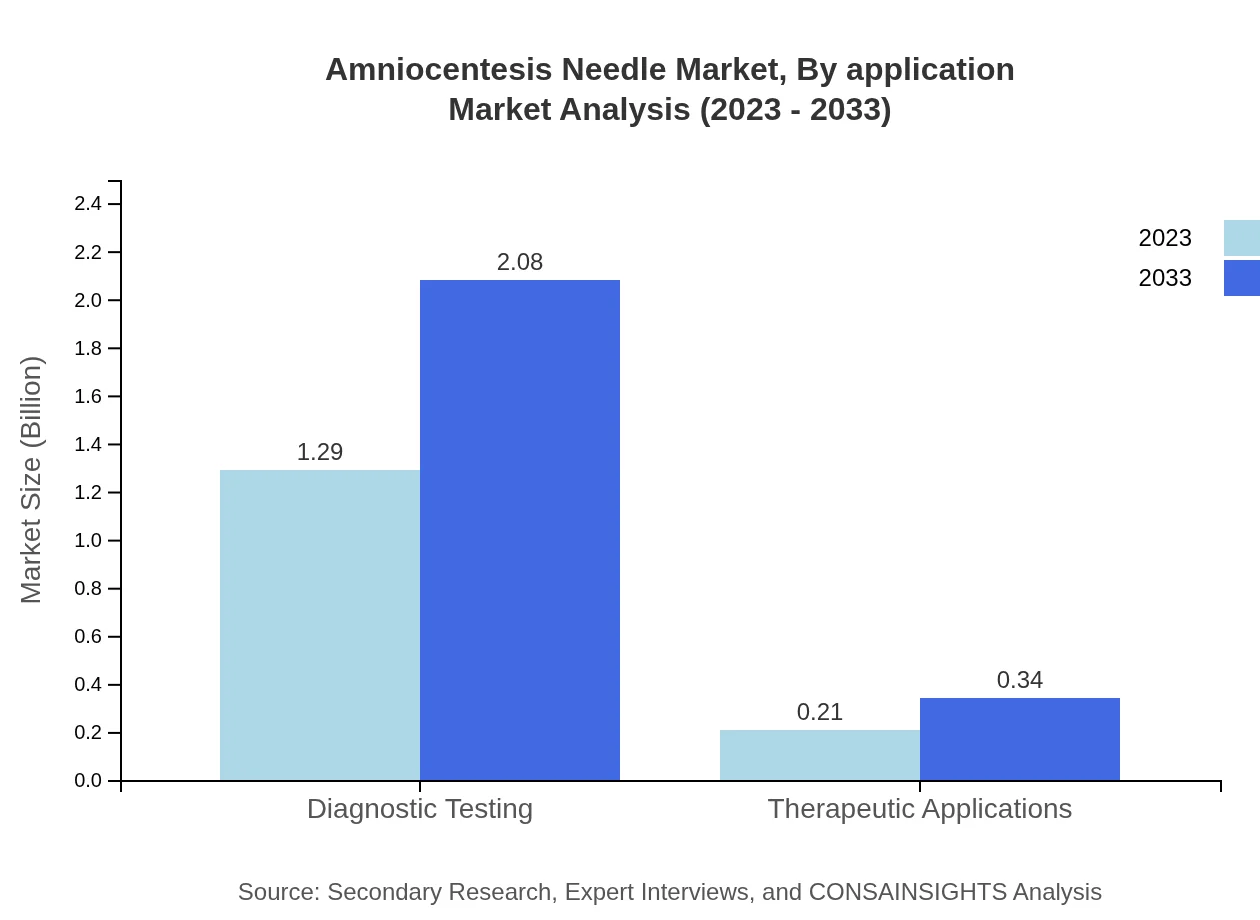
Amniocentesis Needle Market Analysis By Application
The applications of Amniocentesis Needles are classified into diagnostic testing and therapeutic applications. In 2023, the diagnostic testing segment accounted for $1.29 billion, projected to grow to $2.08 billion by 2033, comprising 85.78% of the share. The therapeutic applications segment is estimated to grow from $0.21 billion to $0.34 billion, representing 14.22% of overall market share.
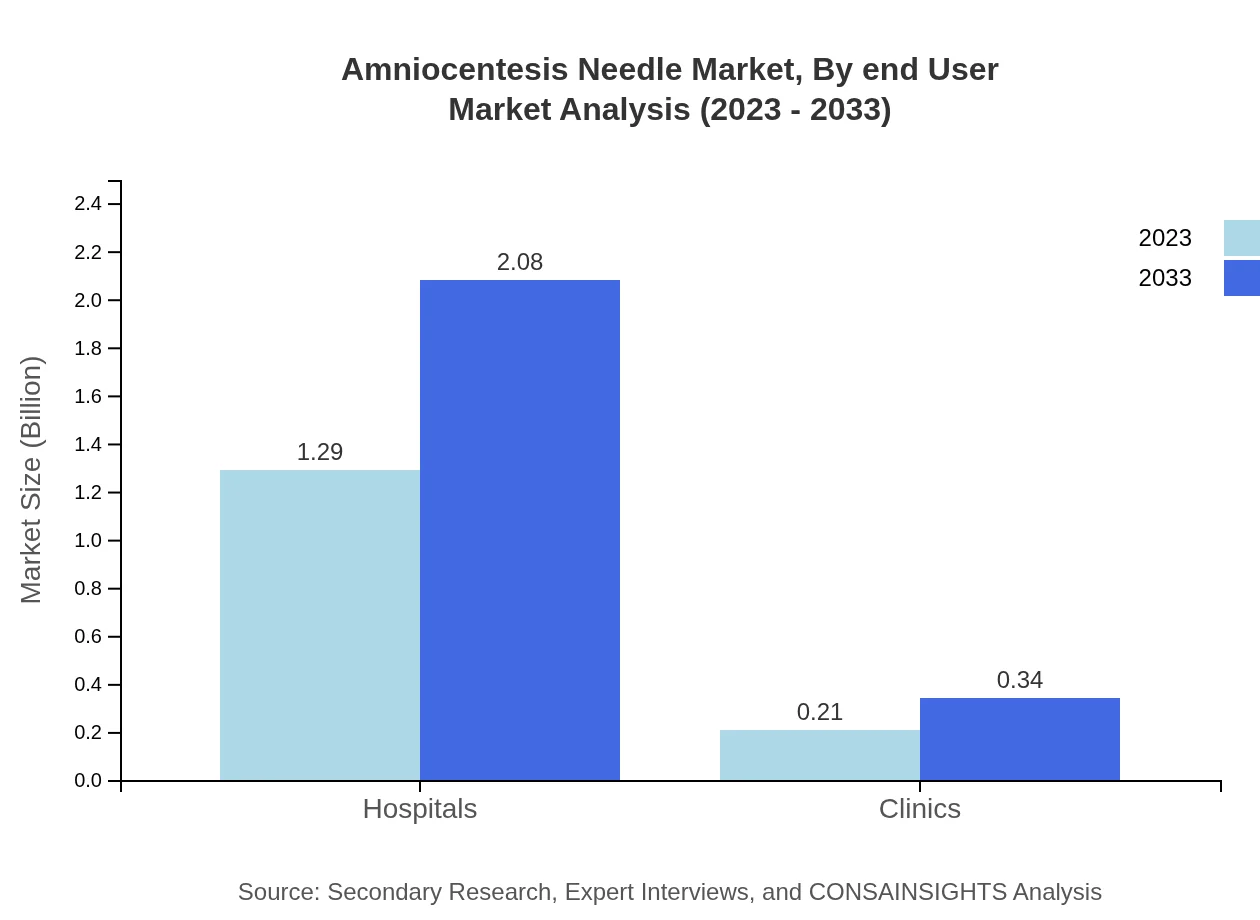
Amniocentesis Needle Market Analysis By End User
Within the end-user segmentation, hospitals are the primary consumers of Amniocentesis Needles, with a market size of $1.29 billion in 2023, expected to rise to $2.08 billion by 2033, capturing 85.78% share. Clinics, while smaller, are expected to grow from $0.21 billion to $0.34 billion, representing 14.22% of the market share.
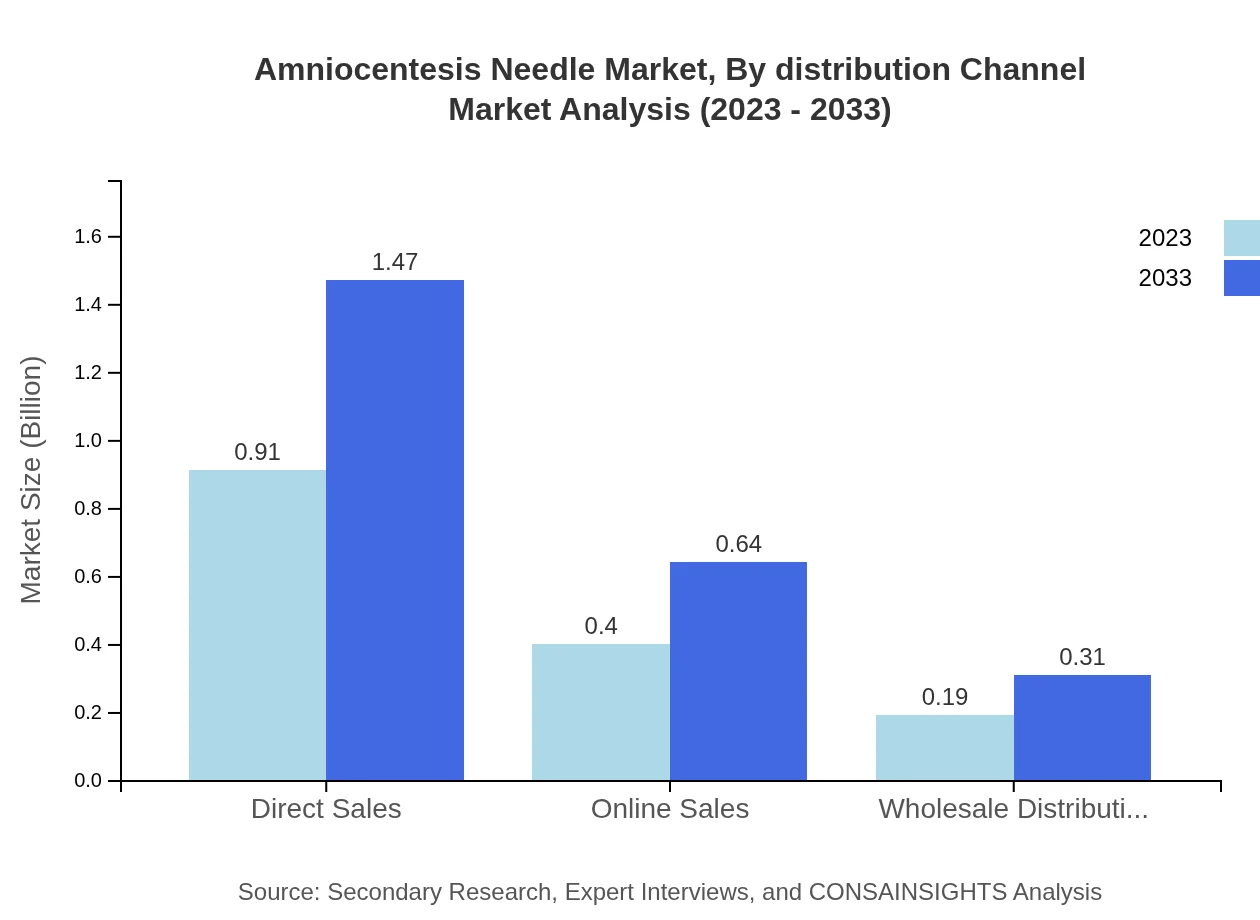
Amniocentesis Needle Market Analysis By Distribution Channel
Distribution channels include direct sales, online sales, and wholesale distribution. Direct sales lead the channel with a size of $0.91 billion in 2023, projected to climb to $1.47 billion by 2033, maintaining a share of 60.84%. Online sales, while emerging, are expected to grow from $0.40 billion to $0.64 billion, commanding a 26.54% market share. Wholesale distribution contributes a smaller segment, from $0.19 billion to $0.31 billion, which makes up 12.62%.
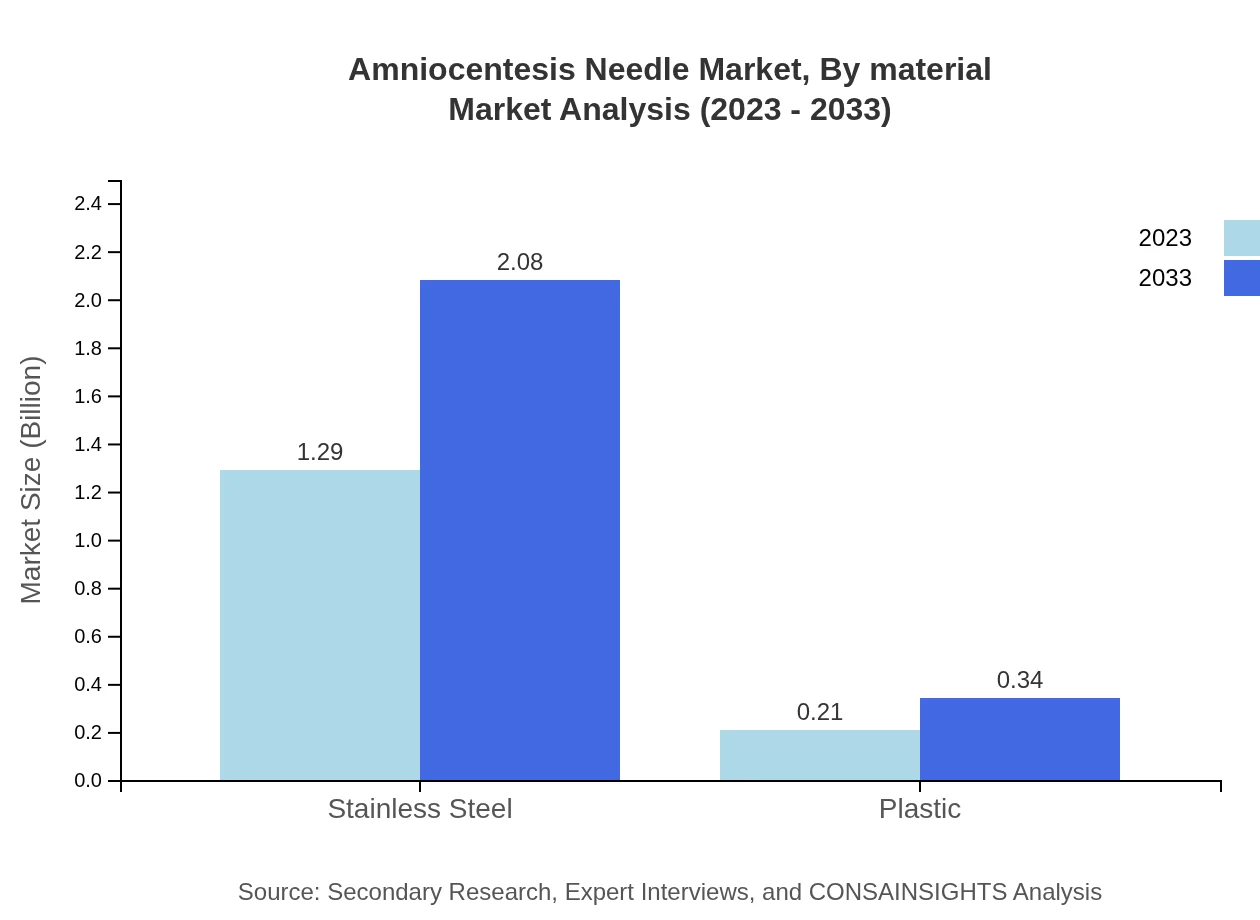
Amniocentesis Needle Market Analysis By Material
Materials utilized in manufacturing Amniocentesis Needles are crucial for procedural safety and effectiveness. The market is segmented into stainless steel and plastic. Stainless steel needles dominate with an estimated market size of $1.29 billion in 2023, projected to grow to $2.08 billion by 2033, holding an 85.78% share. Plastic needles, although less common, are expected to increase from $0.21 billion to $0.34 billion, capturing 14.22% of the market.
Amniocentesis Needle Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Amniocentesis Needle Industry
Medtronic :
Medtronic is a global leader in medical equipment, providing innovative solutions in prenatal diagnostics, including advanced amniocentesis needles that enhance precision and safety in procedures.Becton, Dickinson and Company (BD):
BD is well-known for its commitment to research and development in medical instruments. Rapidly becoming a prominent supplier of amniocentesis needles, BD focuses on improving patient experience and clinical outcomes.Cook Medical:
Cook Medical specializes in a range of medical devices, providing high-quality amniocentesis needles tailored to enhance procedural safety and effectiveness, serving hospitals and clinics worldwide.We're grateful to work with incredible clients.









FAQs
What is the market size of amniocentesis Needle?
The amniocentesis needle market is valued at approximately $1.5 billion in 2023, with a projected CAGR of 4.8%. This growth indicates a steady demand for amniocentesis procedures over the next decade, with significant implications for healthcare stakeholders.
What are the key market players or companies in this amniocentesis Needle industry?
Key players in the amniocentesis needle market include major medical device manufacturers and biotechnology companies. These organizations focus on innovation, quality, and distribution channels to maintain competitive advantages in the global market.
What are the primary factors driving the growth in the amniocentesis needle industry?
The growth in the amniocentesis needle market is driven by increased awareness of prenatal diagnostics, advancements in medical technology, rising birth rates, and a growing demand for patient-centric healthcare solutions, all contributing to higher adoption of these procedures.
Which region is the fastest Growing in the amniocentesis needle market?
The fastest-growing region in the amniocentesis needle market is North America, projected to grow from $0.57 billion in 2023 to $0.92 billion by 2033. This growth is attributed to advanced healthcare infrastructure and a high prevalence of prenatal testing.
Does ConsaInsights provide customized market report data for the amniocentesis needle industry?
Yes, ConsaInsights offers customized market report data for the amniocentesis needle industry. This includes tailored insights based on specific client needs, product segments, and regional markets to facilitate informed decision-making.
What deliverables can I expect from this amniocentesis needle market research project?
Deliverables from the amniocentesis needle market research project include comprehensive market analysis, segment data, regional insights, competitive landscape assessments, and tailored recommendations designed to support strategic business initiatives.
What are the market trends of amniocentesis needle?
Current market trends in the amniocentesis needle space include a shift towards single-use needles, increased focus on non-invasive prenatal testing, and advancements in needle technology that enhance safety and efficacy in medical applications.