Amniotic Products Market Report
Published Date: 31 January 2026 | Report Code: amniotic-products
Amniotic Products Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Amniotic Products market from 2023 to 2033, covering pivotal insights on market size, growth trends, regional dynamics, and segmentation. It aims to equip stakeholders with the crucial data necessary for informed decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
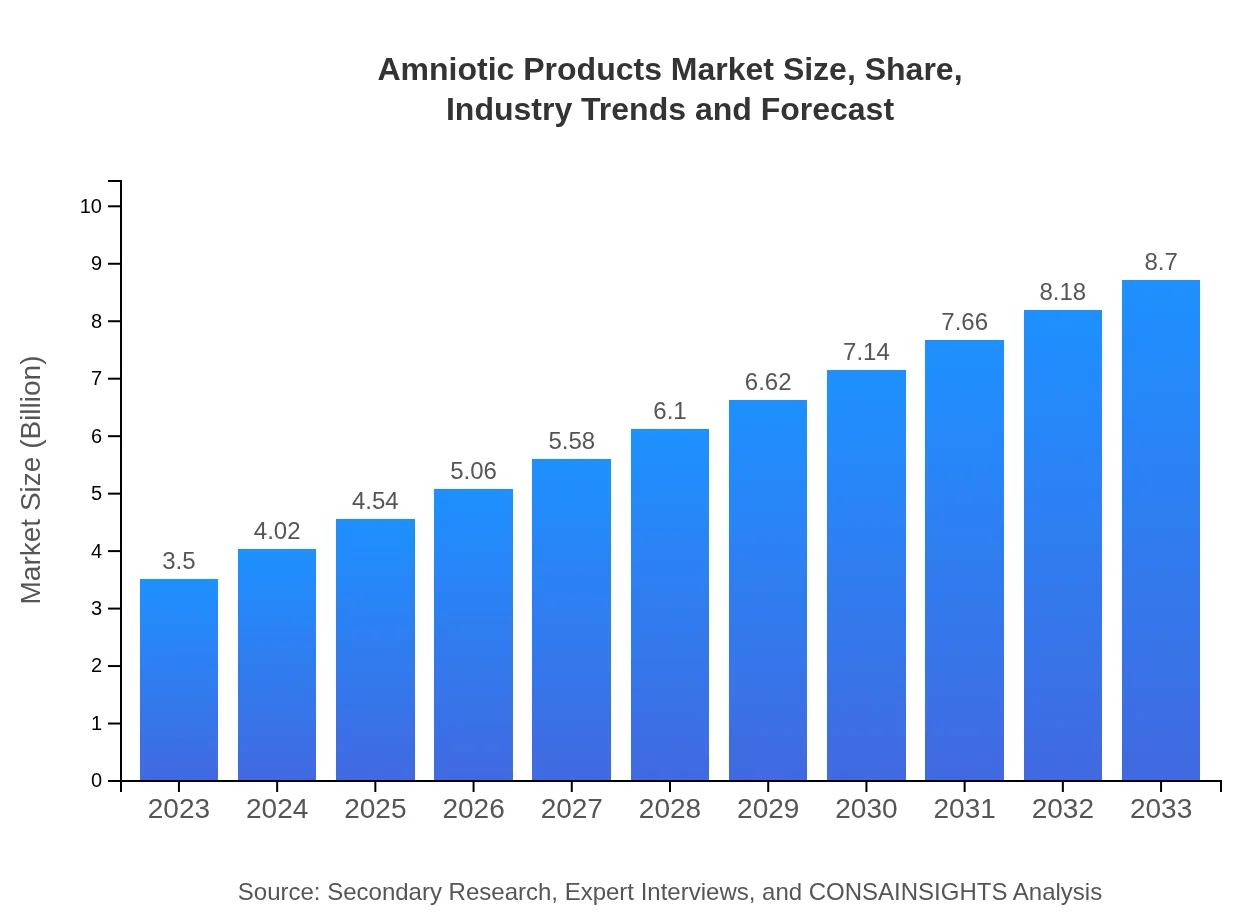
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 9.2% |
| 2033 Market Size | $8.70 Billion |
| Top Companies | Amniox Medical, Inc., MiMedx Group, Inc., Regenative Labs, LLC., SkinMedica, Inc. |
| Last Modified Date | 31 January 2026 |
Amniotic Products Market Overview
Customize Amniotic Products Market Report market research report
- ✔ Get in-depth analysis of Amniotic Products market size, growth, and forecasts.
- ✔ Understand Amniotic Products's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Amniotic Products
What is the Market Size & CAGR of Amniotic Products market in 2023?
Amniotic Products Industry Analysis
Amniotic Products Market Segmentation and Scope
Tell us your focus area and get a customized research report.
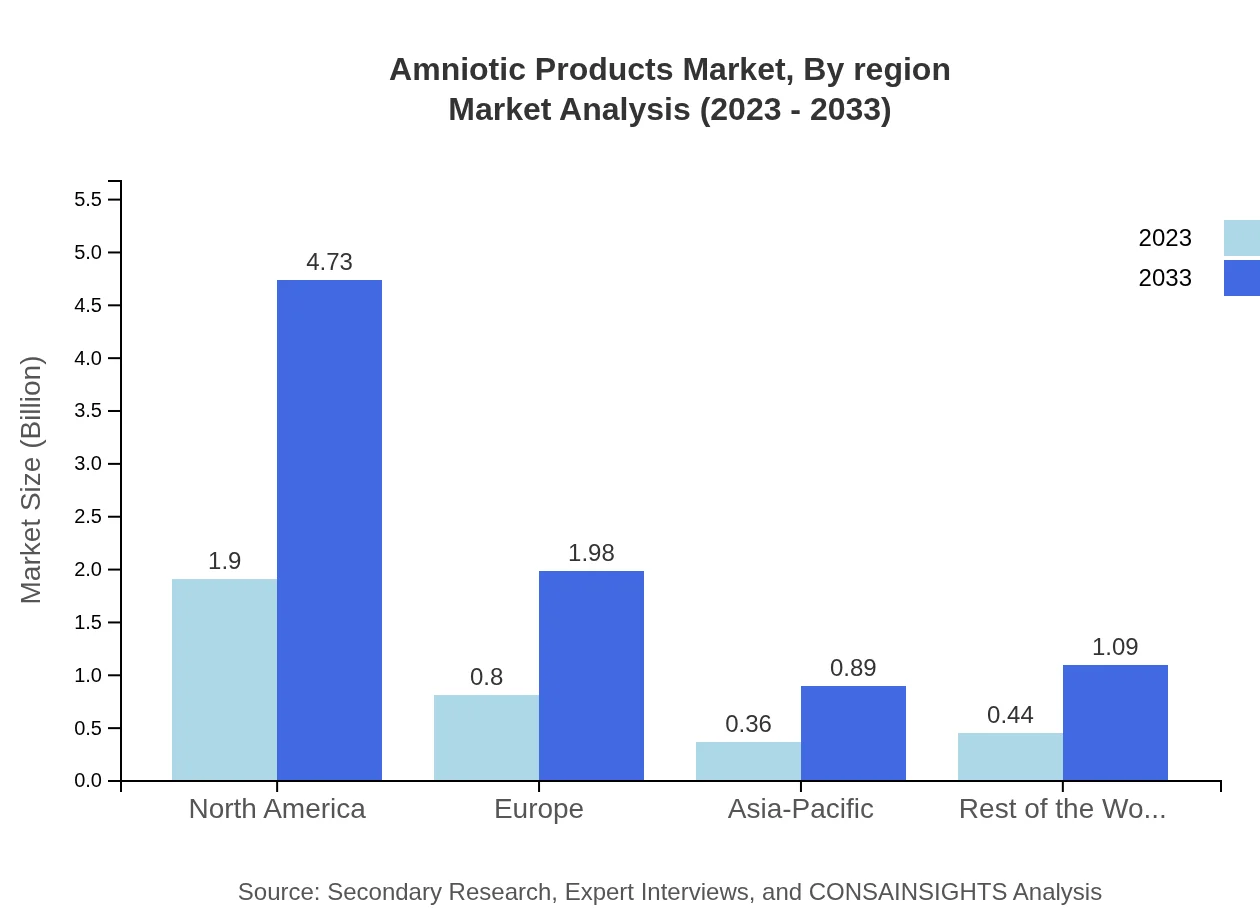
Amniotic Products Market Analysis Report by Region
Europe Amniotic Products Market Report:
The European Amniotic Products market is forecasted to grow from USD 1.13 billion in 2023 to USD 2.82 billion by 2033. The strong focus on regenerative medicine and substantial investments in healthcare are key drivers of growth within this region.Asia Pacific Amniotic Products Market Report:
The Asia-Pacific region is anticipated to witness robust growth, with the market size projected to reach USD 1.37 billion by 2033, up from USD 0.55 billion in 2023. The increasing healthcare infrastructure and rising disposable income levels contribute to this growth, alongside an increasing awareness of advanced surgical solutions.North America Amniotic Products Market Report:
North America dominates the global Amniotic Products market, with a size of USD 1.29 billion in 2023 projected to grow to USD 3.21 billion by 2033. High healthcare expenditure, extensive research activities, and early adoption of advanced medical technologies bolster market growth in this region.South America Amniotic Products Market Report:
South America is expected to experience steady growth, with the market size projected to increase from USD 0.26 billion in 2023 to USD 0.65 billion by 2033. Factors such as the rising incidence of chronic conditions and an evolving healthcare system are driving demand for innovative therapeutic options.Middle East & Africa Amniotic Products Market Report:
The Middle East and Africa region is set to see a gradual increase, with the market increasing from USD 0.26 billion in 2023 to USD 0.65 billion by 2033. Government initiatives and improved healthcare facilities are likely to fuel market development in this region.Tell us your focus area and get a customized research report.
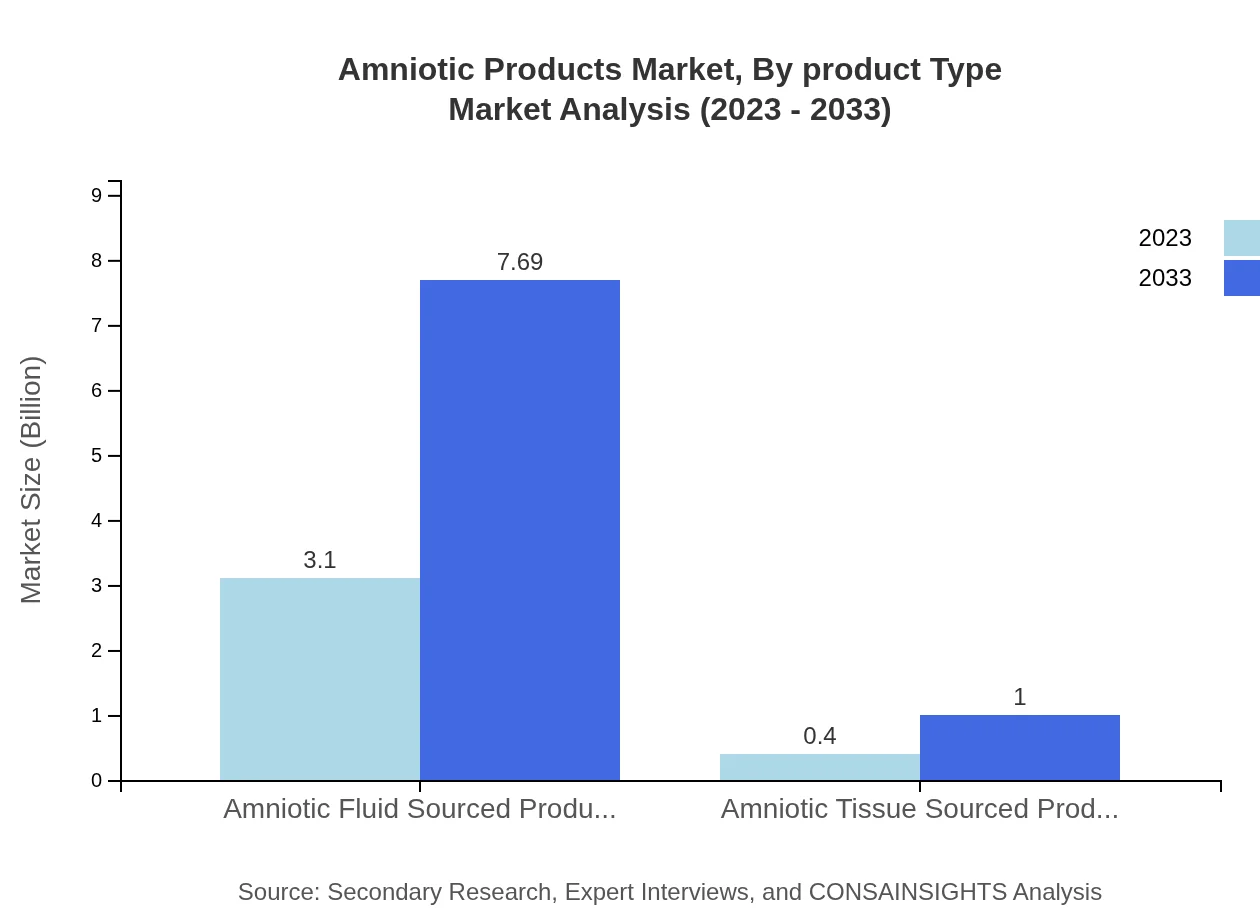
Amniotic Products Market Analysis By Product Type
Amniotic Products are divided into amniotic fluid sourced and amniotic tissue sourced categories. The former holds a considerable market share of 88.46% by revenue, attributed to its extensive use in various medical applications. The tissue-sourced products, however, contribute 11.54% of the market, primarily utilized in surgical and regenerative medicine due to their unique properties.
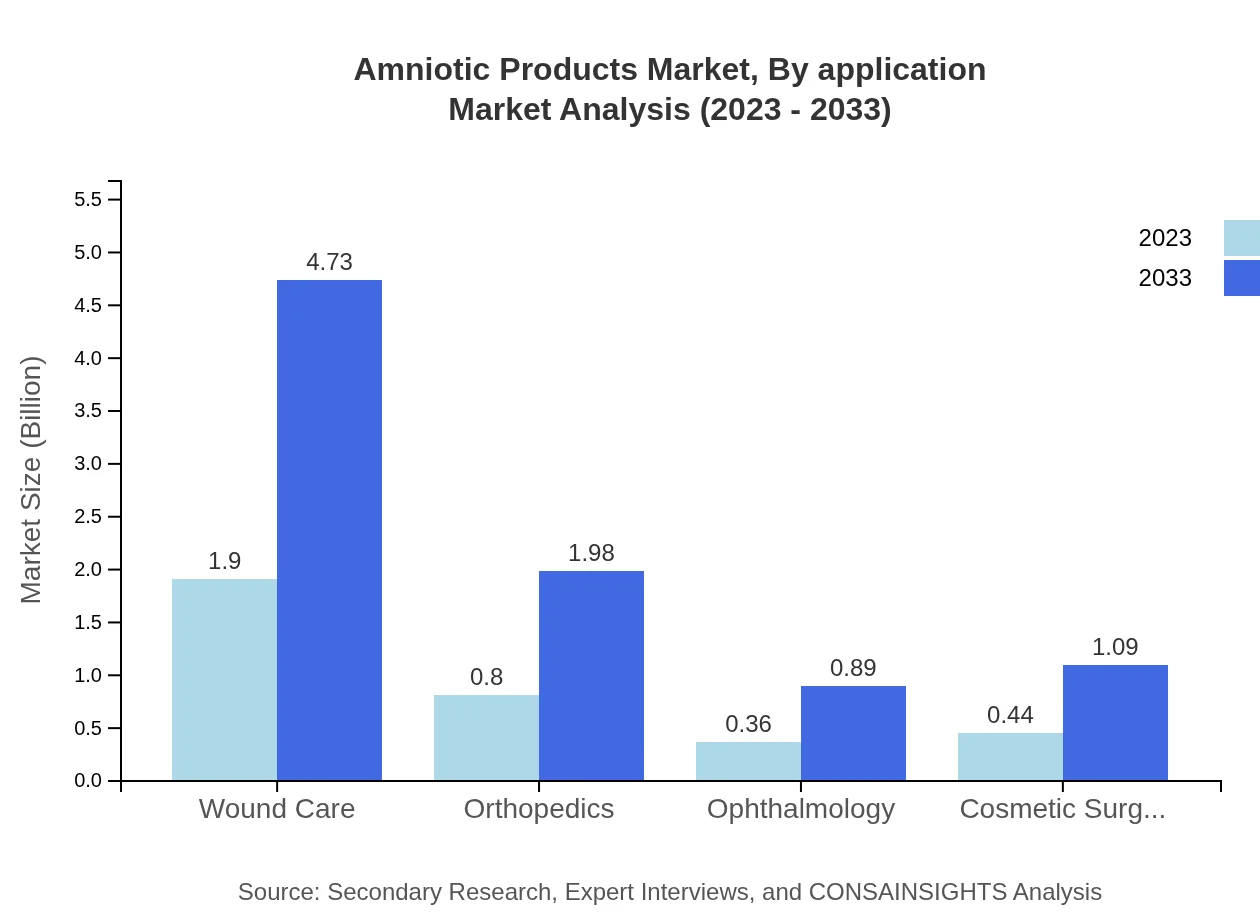
Amniotic Products Market Analysis By Application
Applications encompass wound care, orthopedics, ophthalmology, and cosmetic surgery. Wound care applications yield a significant market share (54.39%), followed by orthopedics (22.81%), reflecting the versatile use of amniotic products in healing and tissue repair. The ophthalmology and cosmetic surgery segments also demonstrate promising growth, capitalizing on advancements in minimally invasive procedures.
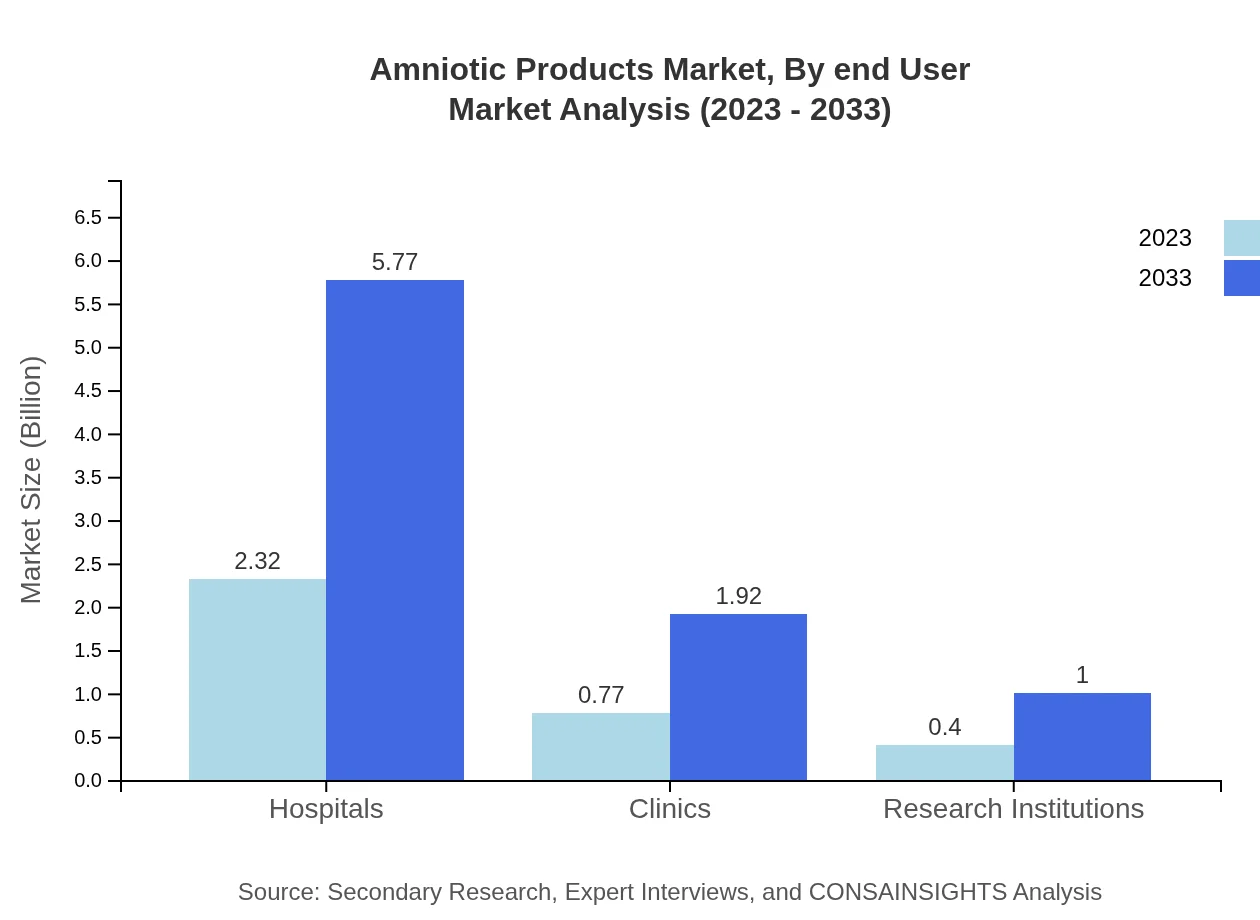
Amniotic Products Market Analysis By End User
End-user segments include hospitals, clinics, and research institutions. Hospitals dominate the market with a 66.39% share, attributed to extensive surgical applications. Clinics are responsible for 22.06% and are a growing channel as outpatient procedures increase. Research institutions are gaining traction as they explore novel therapeutic applications of amniotic products, holding an 11.55% market share.
Amniotic Products Market Analysis By Region
Regional analysis showcases North America leading the market with a significant share of 54.39%, followed by Europe at 22.81% and the Asia-Pacific region at 10.28%. Emerging markets like South America and the Middle East and Africa are progressively capturing attention due to expanding healthcare initiatives.
Amniotic Products Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Amniotic Products Industry
Amniox Medical, Inc.:
Amniox specializes in regenerative medicine products derived from amniotic membrane and fluid, helping in wound healing and surgical applications.MiMedx Group, Inc.:
A leading developer of regenerative medicine products, MiMedx focuses on amniotic tissue allografts for various surgical and wound care applications.Regenative Labs, LLC.:
Regenative Labs produces amniotic allografts and focuses on innovative solutions for orthopedic and surgical applications.SkinMedica, Inc.:
SkinMedica is known for its skincare products utilizing amniotic tissue for sophisticated regenerative therapies in dermatology.We're grateful to work with incredible clients.









FAQs
What is the market size of amniotic Products?
The amniotic products market is valued at approximately $3.5 billion in 2023, with a robust CAGR of 9.2% projected through 2033. This growth reflects the increasing application of amniotic products in various medical fields, boosting market potential.
What are the key market players or companies in this amniotic Products industry?
Key players in the amniotic products market include prominent biotech firms and regenerative medicine companies, specializing in amniotic membrane technologies and other related innovations. These companies drive advances in application methodologies, enhancing treatment options and outcomes.
What are the primary factors driving the growth in the amniotic Products industry?
Growth in the amniotic products industry is driven by increasing adoption in wound care and orthopedics, advancements in regenerative medicine, and rising awareness of the therapeutic benefits of amniotic tissue. These factors collectively fuel innovation and market demand.
Which region is the fastest Growing in the amniotic Products?
The fastest-growing region in the amniotic products market is North America, with a market size projected to rise from $1.29 billion in 2023 to $3.21 billion by 2033, reflecting significant investment in healthcare technologies and extensive clinical applications.
Does ConsaInsights provide customized market report data for the amniotic Products industry?
Yes, ConsaInsights offers tailored market reports for the amniotic products industry, allowing clients to obtain specific data and insights based on unique business needs. This customization facilitates informed decision-making and strategic planning.
What deliverables can I expect from this amniotic Products market research project?
Deliverables from the amniotic products market research project include comprehensive reports, market forecasts, competitive analyses, and insights into trends and emerging opportunities. Clients receive detailed data to support strategic initiatives.
What are the market trends of amniotic Products?
Current trends in the amniotic products market include an increased focus on regenerative medicine, higher demand for minimally invasive procedures, and the expansion of applications in specialized surgeries. These trends signify an evolving healthcare landscape.