Anatomic Pathology Track And Trace Solutions Market Report
Published Date: 31 January 2026 | Report Code: anatomic-pathology-track-and-trace-solutions
Anatomic Pathology Track And Trace Solutions Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Anatomic Pathology Track And Trace Solutions market from 2023 to 2033, including insights into market size, trends, and growth prospects across various regions and segments.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
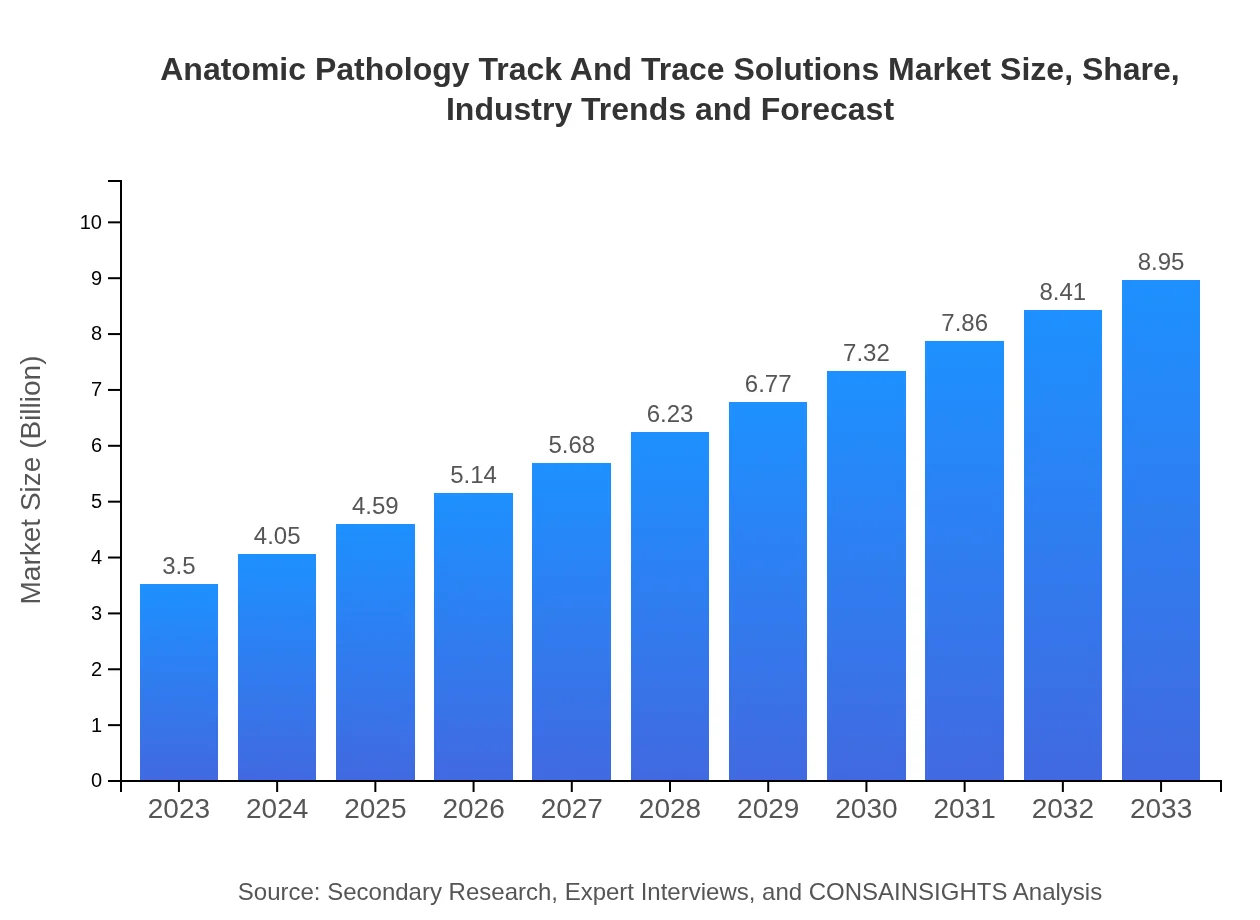
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 9.5% |
| 2033 Market Size | $8.95 Billion |
| Top Companies | Thermo Fisher Scientific, Siemens Healthineers, LabVantage Solutions |
| Last Modified Date | 31 January 2026 |
Anatomic Pathology Track And Trace Solutions Market Overview
Customize Anatomic Pathology Track And Trace Solutions Market Report market research report
- ✔ Get in-depth analysis of Anatomic Pathology Track And Trace Solutions market size, growth, and forecasts.
- ✔ Understand Anatomic Pathology Track And Trace Solutions's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Anatomic Pathology Track And Trace Solutions
What is the Market Size & CAGR of Anatomic Pathology Track And Trace Solutions market in {Year}?
Anatomic Pathology Track And Trace Solutions Industry Analysis
Anatomic Pathology Track And Trace Solutions Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Anatomic Pathology Track And Trace Solutions Market Analysis Report by Region
Europe Anatomic Pathology Track And Trace Solutions Market Report:
Europe's market is set to expand from $0.99 billion in 2023 to $2.53 billion by 2033, thanks to stringent regulations and the established presence of leading market players in the region.Asia Pacific Anatomic Pathology Track And Trace Solutions Market Report:
The Asia Pacific region is projected to grow from $0.65 billion in 2023 to $1.67 billion by 2033, with an increasing focus on enhancing healthcare infrastructure and technological adoption in countries like China and India.North America Anatomic Pathology Track And Trace Solutions Market Report:
North America remains the largest market for Anatomic Pathology Track And Trace Solutions, expected to grow from $1.35 billion in 2023 to $3.44 billion by 2033, owing to advanced healthcare systems and high investment in innovation.South America Anatomic Pathology Track And Trace Solutions Market Report:
In South America, the market is expected to increase from $0.15 billion in 2023 to $0.38 billion by 2033, driven by rising healthcare investments and growing awareness about laboratory safety.Middle East & Africa Anatomic Pathology Track And Trace Solutions Market Report:
The Middle East and Africa market is projected to grow from $0.36 billion in 2023 to $0.93 billion by 2033, as countries enhance their healthcare services amid economic growth and urbanization.Tell us your focus area and get a customized research report.
Anatomic Pathology Track And Trace Solutions Market Analysis By Product
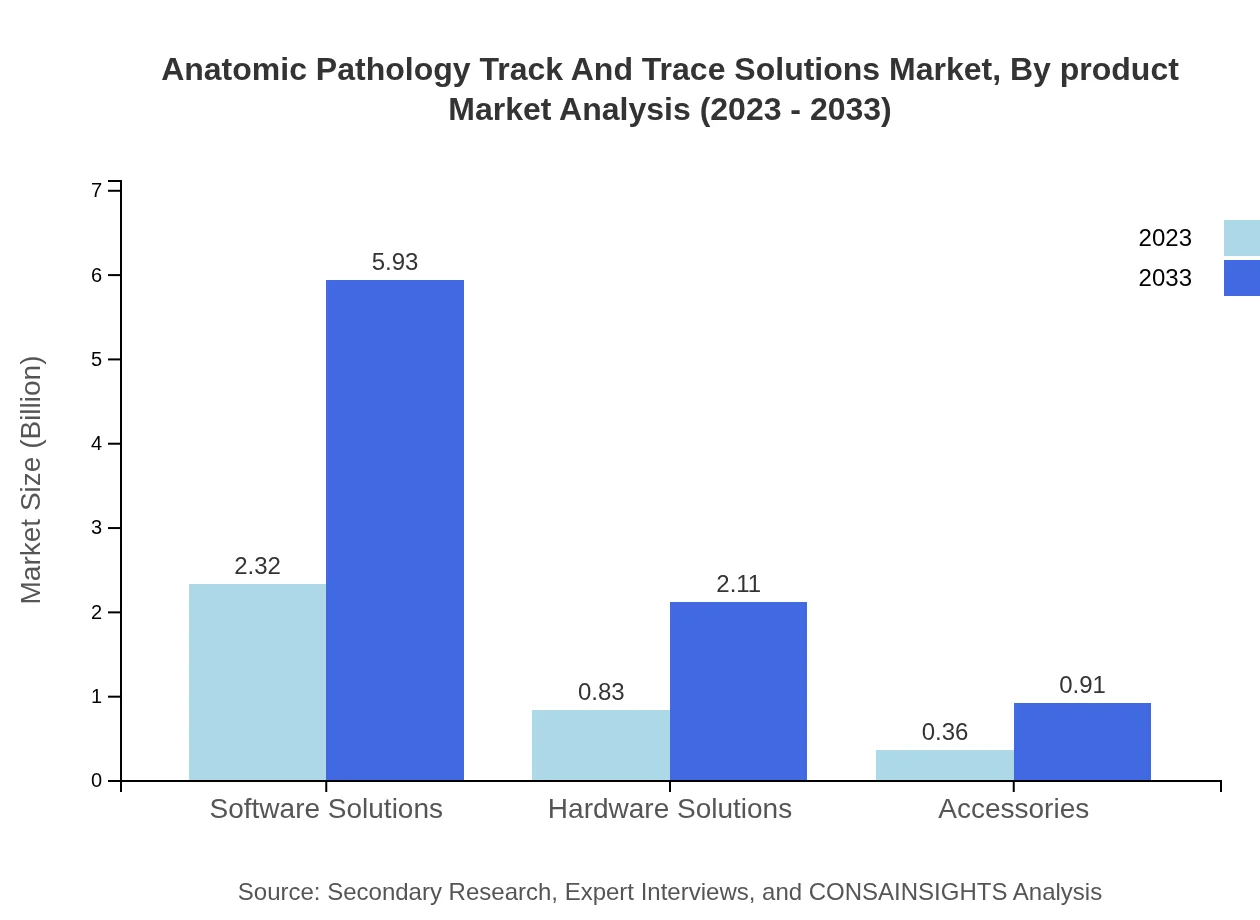
The software solutions segment leads the market, demonstrating a size of $2.32 billion in 2023 and projected to rise to $5.93 billion by 2033, with a consistent market share of 66.19%. Hardware solutions, valued at $0.83 billion in 2023, will increase to $2.11 billion, maintaining a 23.62% share. Accessories currently hold a smaller piece, ranging from $0.36 billion in 2023 to $0.91 billion by 2033.
Anatomic Pathology Track And Trace Solutions Market Analysis By Application
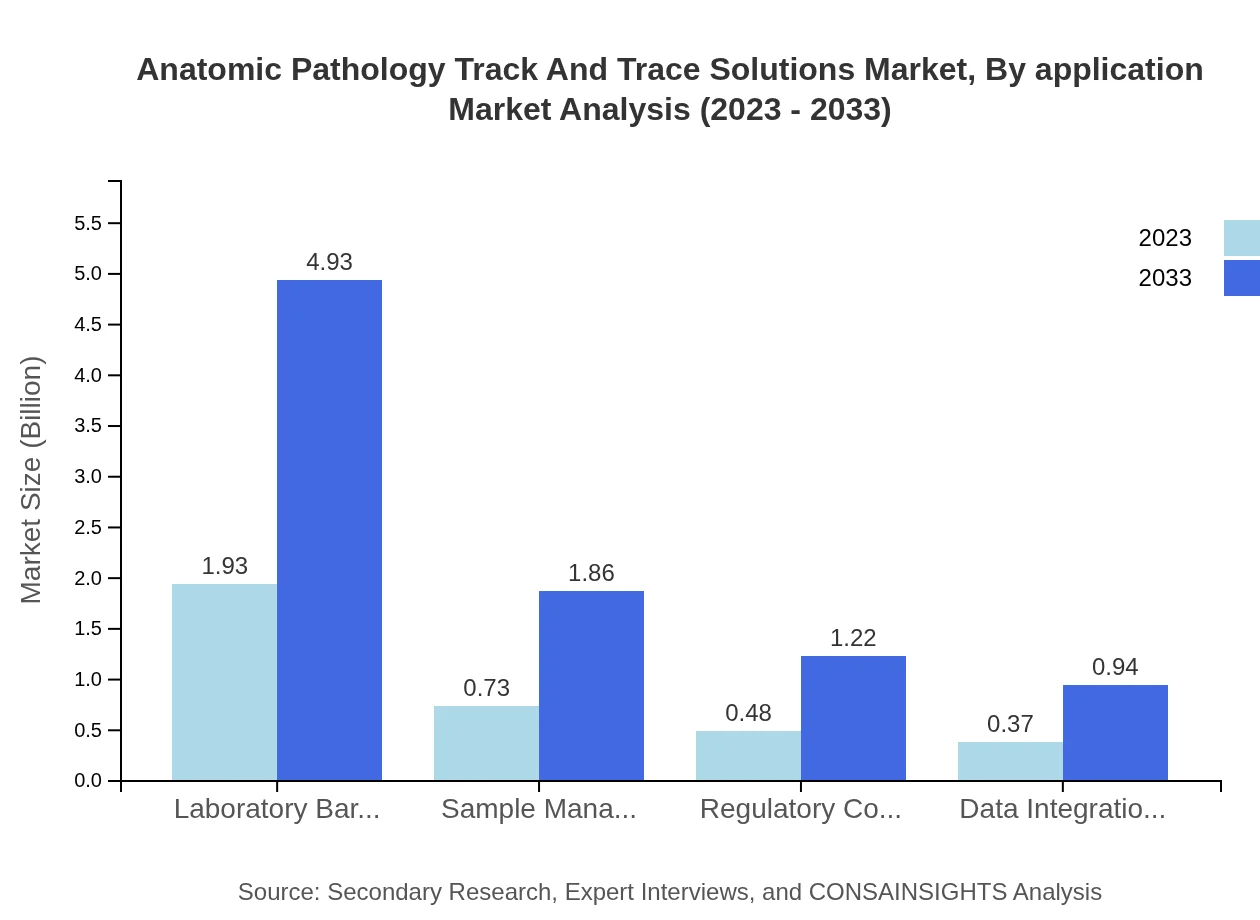
Hospitals occupy a significant portion of the market, quantified at $2.32 billion in 2023, growing to $5.93 billion by 2033, retaining a 66.19% share. Pathology laboratories are expected to grow from $0.83 billion to $2.11 billion, with a 23.62% share. Research institutions will experience growth from $0.36 billion in 2023 to $0.91 billion by 2033.
Anatomic Pathology Track And Trace Solutions Market Analysis By End User
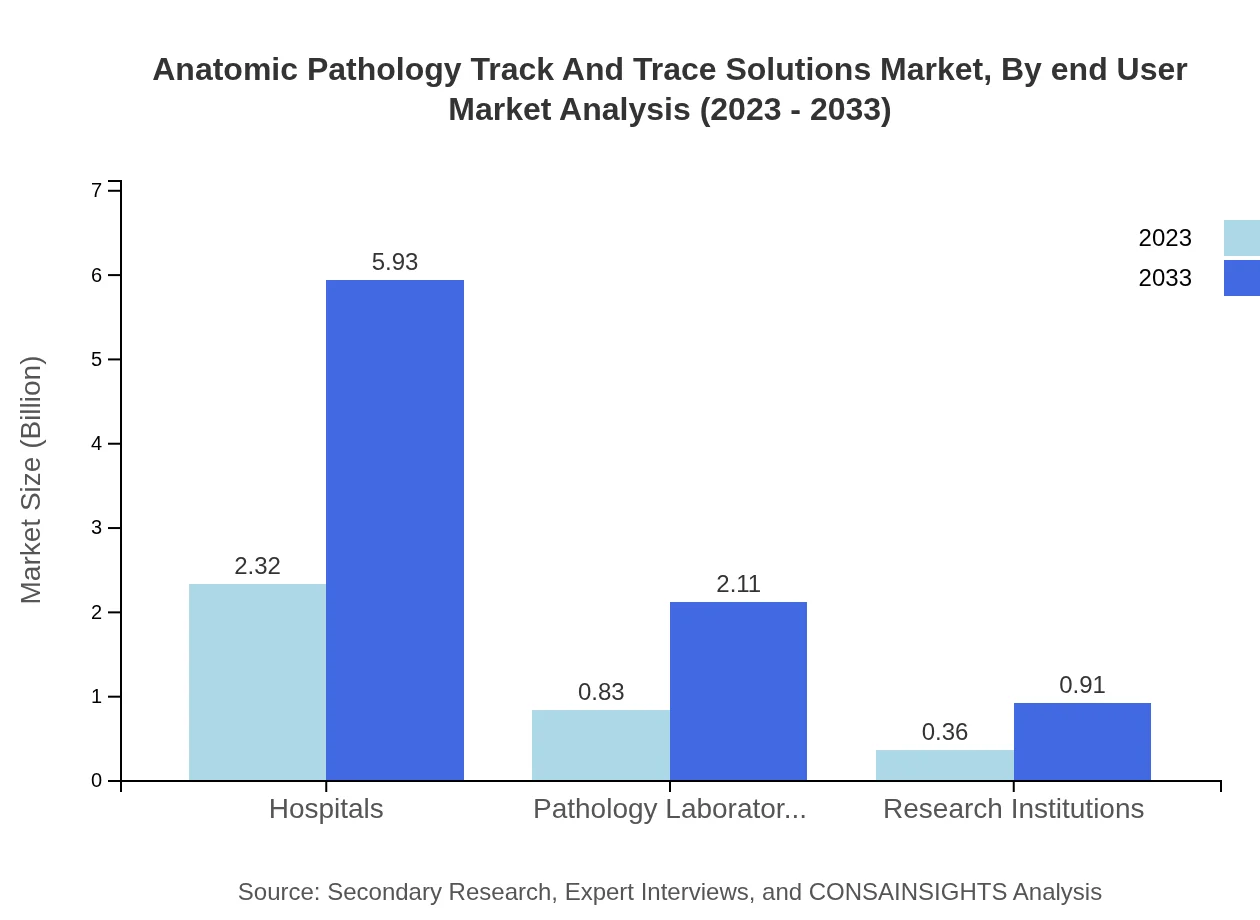
The end-users are mainly hospitals, pathology laboratories, and research institutions. Hospitals and pathology laboratories significantly drive the market demand due to increasing specimen volumes, while research institutions enhance the scope through innovation and development.
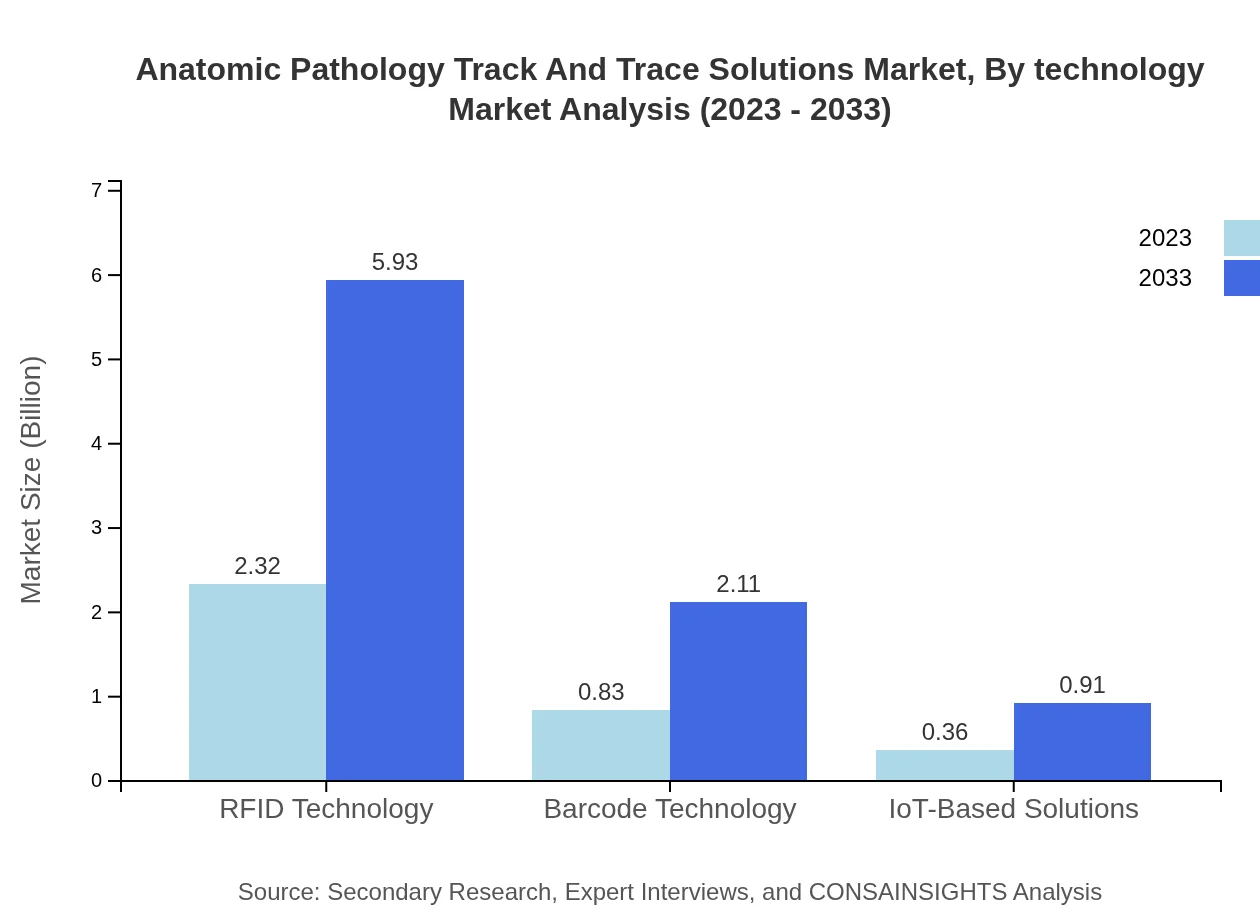
Anatomic Pathology Track And Trace Solutions Market Analysis By Technology
The technology landscape in this market is dominated by RFID technology, projected to grow from $2.32 billion in 2023 to $5.93 billion by 2033, capturing 66.19% market share. Following closely is Barcode technology, expected to increase from $0.83 billion to $2.11 billion, and IoT-Based Solutions, growing from $0.36 billion to $0.91 billion.
Anatomic Pathology Track And Trace Solutions Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Anatomic Pathology Track And Trace Solutions Industry
Thermo Fisher Scientific:
A leader in scientific instrumentation and laboratory services, Thermo Fisher offers a range of solutions enhancing the tracking of specimens across laboratories.Siemens Healthineers:
Known for digital transformation in healthcare, Siemens Healthineers provides innovative solutions for managing pathologic processes and ensuring specimen traceability.LabVantage Solutions:
A key player specializing in LIMS solutions, LabVantage supports laboratories in managing specimen tracking, thereby improving overall laboratory productivity.We're grateful to work with incredible clients.









FAQs
What is the market size of Anatomic Pathology Track and Trace Solutions?
The global market size for Anatomic Pathology Track and Trace Solutions is projected to reach $3.5 billion by 2033, growing at a CAGR of 9.5% from 2023. This growth reflects the increasing need for efficient tracking and management in the healthcare sector.
What are the key market players or companies in the Anatomic Pathology Track and Trace Solutions industry?
Key players in the Anatomic Pathology Track and Trace Solutions market include leading medical technology companies that focus on software and hardware solutions. These companies innovate in connectivity, data management, and compliance to enhance pathology operations.
What are the primary factors driving the growth in the Anatomic Pathology Track and Trace Solutions industry?
Driving factors for growth in this industry include technological advancements in tracking solutions, rising demand for efficient sample management, and the need for regulatory compliance in laboratories, which enhance workflow efficiency and patient safety.
Which region is the fastest Growing in the Anatomic Pathology Track and Trace Solutions?
The fastest-growing region in the Anatomic Pathology Track and Trace Solutions market is North America, with a market size of $3.44 billion projected by 2033. This growth is driven by high healthcare expenditure and innovation in medical technologies.
Does ConsaInsights provide customized market report data for the Anatomic Pathology Track and Trace Solutions industry?
Yes, ConsaInsights offers customized market report data tailored to specific aspects of the Anatomic Pathology Track and Trace Solutions industry. Clients can request specific insights or data segments aligned with their strategic goals.
What deliverables can I expect from this Anatomic Pathology Track and Trace Solutions market research project?
Deliverables from the Anatomic Pathology Track and Trace Solutions market research include detailed market analysis reports, segmentation insights, growth forecasts, competitive landscape evaluations, and recommendations tailored to market trends.
What are the market trends of Anatomic Pathology Track and Trace Solutions?
Current market trends include increasing adoption of software solutions for pathology management, integration of IoT and RFID technologies, and a growing focus on regulatory compliance. These trends reflect the industry's shift towards automation and data-driven decision-making.