Anesthesia Monitoring Devices Market Report
Published Date: 31 January 2026 | Report Code: anesthesia-monitoring-devices
Anesthesia Monitoring Devices Market Size, Share, Industry Trends and Forecast to 2033
This report offers an in-depth analysis of the Anesthesia Monitoring Devices market, showcasing insights from 2023 to 2033, including market trends, size, segmentation, regional dynamics, technology impacts, and forecasts.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
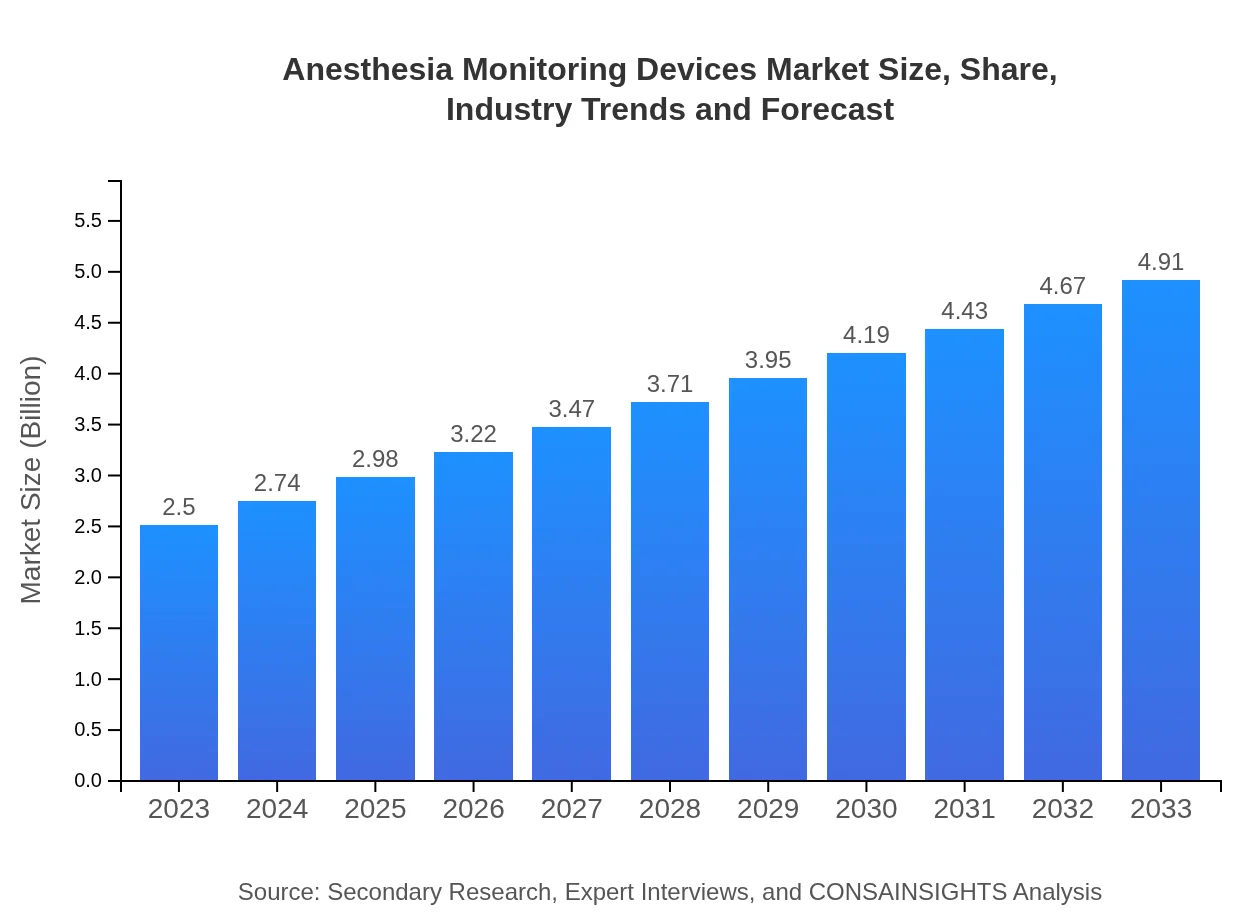
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Medtronic , Philips Healthcare, GE Healthcare, Edwards Lifesciences |
| Last Modified Date | 31 January 2026 |
Anesthesia Monitoring Devices Market Overview
Customize Anesthesia Monitoring Devices Market Report market research report
- ✔ Get in-depth analysis of Anesthesia Monitoring Devices market size, growth, and forecasts.
- ✔ Understand Anesthesia Monitoring Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Anesthesia Monitoring Devices
What is the Market Size & CAGR of Anesthesia Monitoring Devices market in 2023?
Anesthesia Monitoring Devices Industry Analysis
Anesthesia Monitoring Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Anesthesia Monitoring Devices Market Analysis Report by Region
Europe Anesthesia Monitoring Devices Market Report:
In 2023, Europe’s market was valued at approximately USD 0.81 billion, projected to grow to USD 1.58 billion by 2033. The presence of a vast number of ambulatory surgical centers and hospitals and a focus on improving patient safety and outcomes contribute to this growth. Increasing clinical research into anesthetic safety will also drive the demand for monitoring devices.Asia Pacific Anesthesia Monitoring Devices Market Report:
In 2023, the Asia Pacific region's Anesthesia Monitoring Devices market was valued at approximately USD 0.47 billion and is projected to grow to USD 0.92 billion by 2033. The growth is driven by increasing surgical procedures in countries like India and China, coupled with a growing healthcare infrastructure. Emerging markets in this region are also adopting advanced anesthesia monitoring technologies at a faster pace.North America Anesthesia Monitoring Devices Market Report:
North America dominated the Anesthesia Monitoring Devices market with a valuation of USD 0.86 billion in 2023, expected to reach USD 1.69 billion by 2033. Factors such as high healthcare expenditure, advanced healthcare infrastructure, and significant investment in surgical technologies drive growth in this region.South America Anesthesia Monitoring Devices Market Report:
The South American Anesthesia Monitoring Devices market was valued at around USD 0.09 billion in 2023, with an expected increase to USD 0.17 billion by 2033. Rising awareness about patient monitoring during surgeries and improvements in healthcare quality in Brazil and Argentina contribute to this growth. However, economic fluctuations remain a significant challenge.Middle East & Africa Anesthesia Monitoring Devices Market Report:
The Anesthesia Monitoring Devices market in the Middle East and Africa was valued at USD 0.28 billion in 2023, expected to grow to USD 0.55 billion by 2033. The market is expanding due to improving healthcare facilities and growing investments in medical technology. Countries like UAE and South Africa are leading this growth due to increasing surgical interventions.Tell us your focus area and get a customized research report.
Anesthesia Monitoring Devices Market Analysis By End User
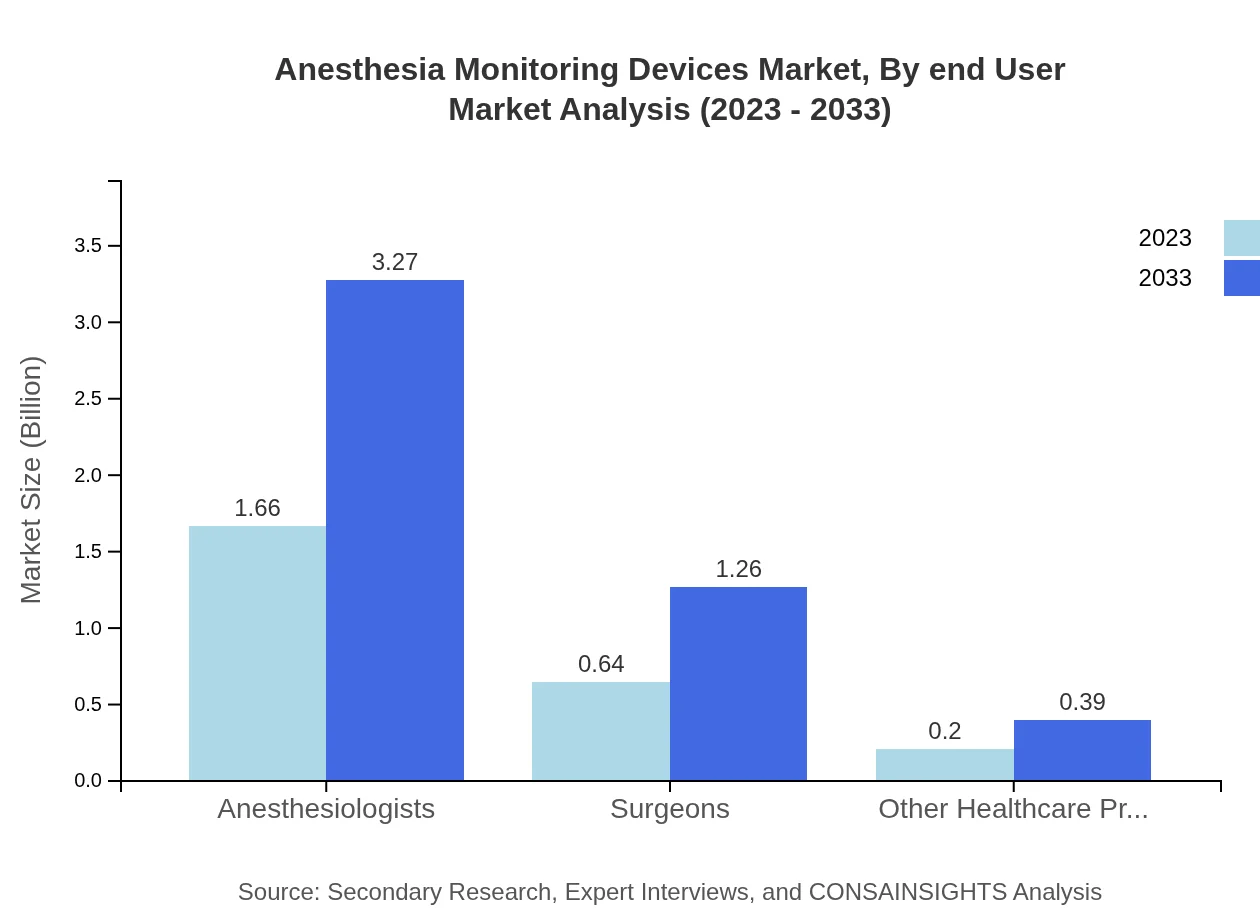
The end-users of Anesthesia Monitoring Devices include hospitals, ambulatory surgery centers, and clinical research institutions. In 2023, hospitals accounted for the largest market share, valued at approximately USD 1.66 billion, and projected to maintain this dominance with a market share of 66.55% by 2033. Ambulatory Surgery Centers also hold significant relevance due to the shift towards outpatient surgery. Their market size is expected to reach USD 1.26 billion by 2033, continuing to account for 25.6% of the market.
Anesthesia Monitoring Devices Market Analysis By Technology
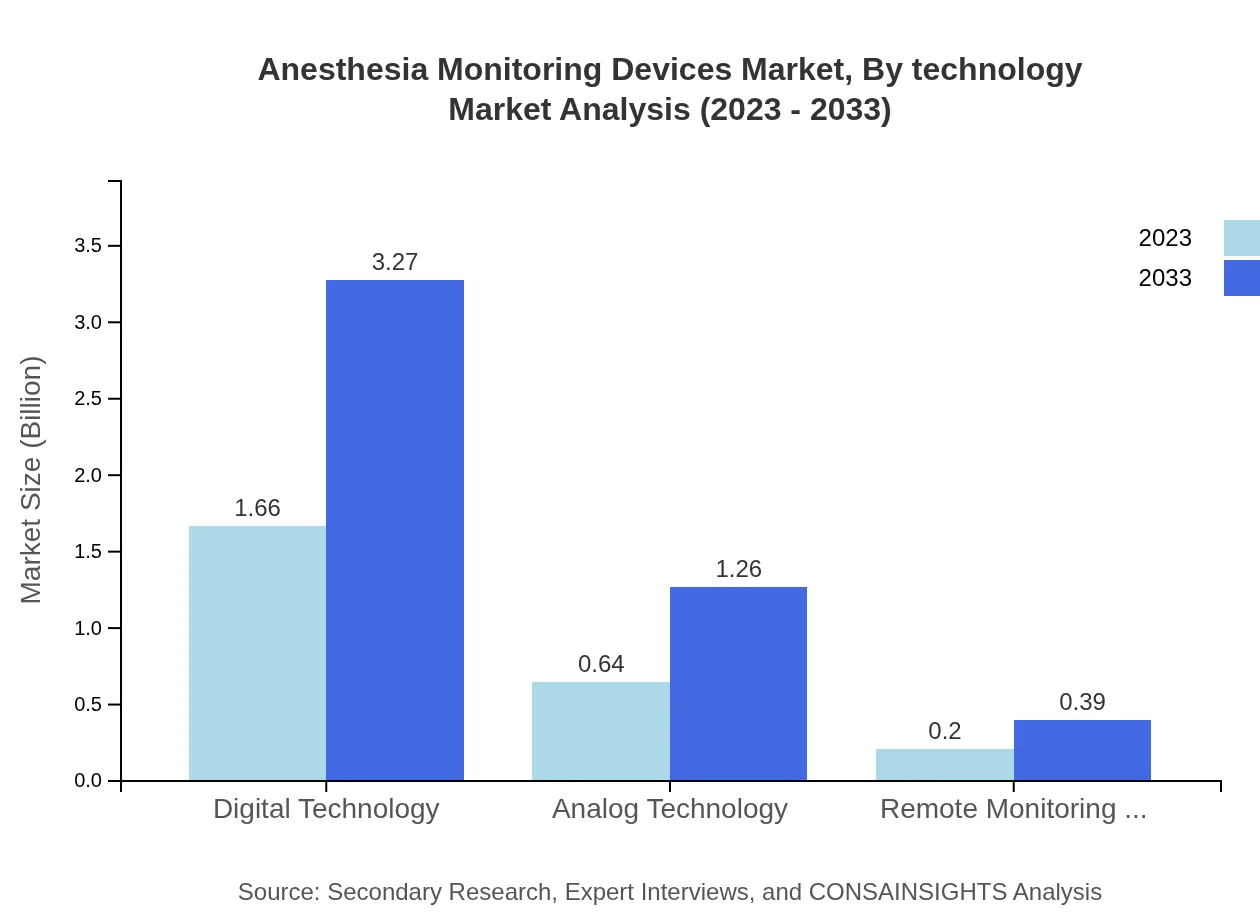
The market for Anesthesia Monitoring Devices is also segmented by technology into digital and analog technologies, alongside remote monitoring technology. The digital technology segment holds the largest market share at 66.55% with expected growth from USD 1.66 billion in 2023 to USD 3.27 billion by 2033. Analog technology, although smaller in share, remains crucial in various applications, projected to encompass USD 1.26 billion by 2033. Remote monitoring technology, growing in popularity, aims at bridging healthcare access gaps and is projected to grow to USD 0.39 billion.
Anesthesia Monitoring Devices Market Analysis By Device Type
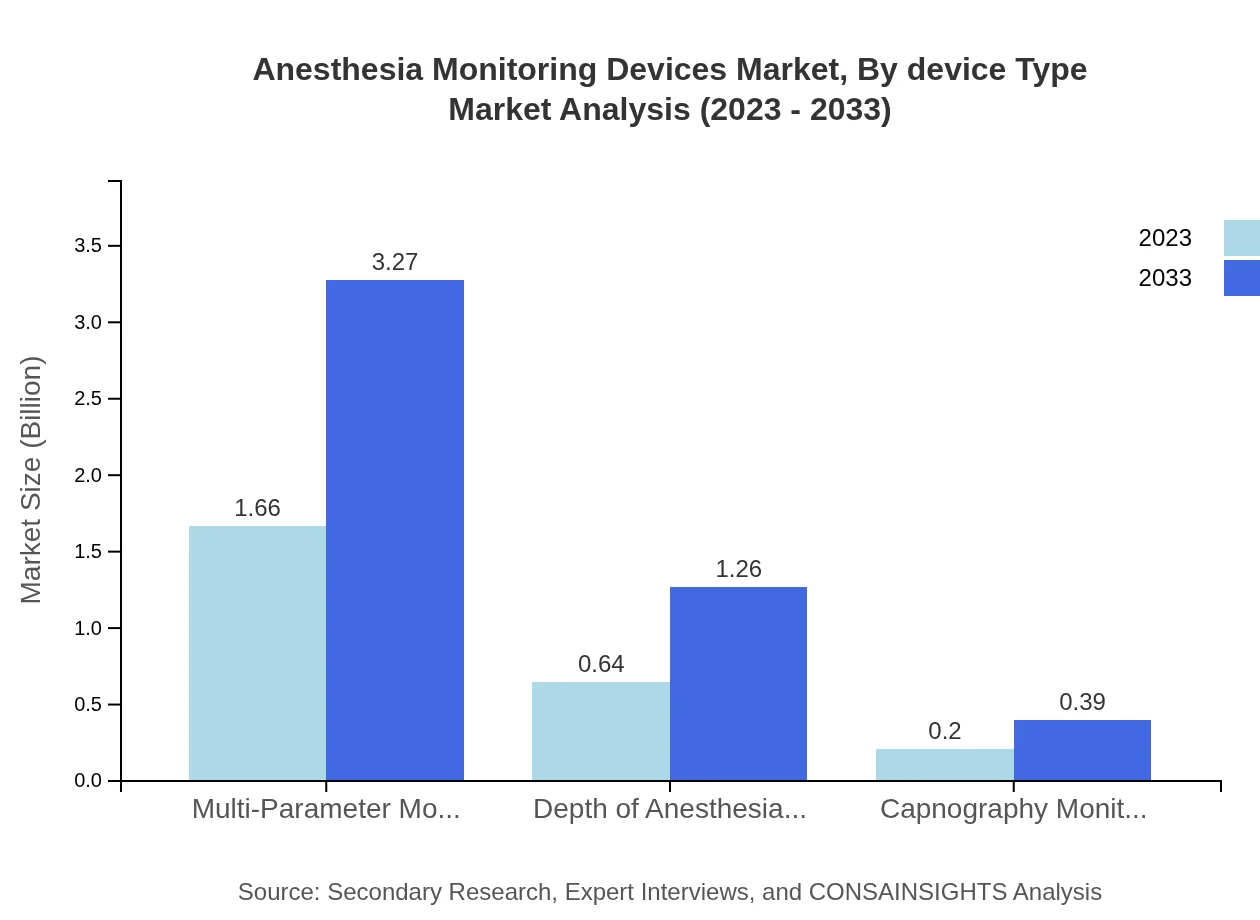
Segments based on device types include Multi-Parameter Monitors, Depth of Anesthesia Monitors, and Capnography Monitors. Multi-Parameter Monitors are leading the market with an expected growth from USD 1.66 billion in 2023 to USD 3.27 billion by 2033, representing 66.55% market share. Depth of Anesthesia Monitors and Capnography Monitors hold relevant portions of the market, expected to reach USD 1.26 billion and USD 0.39 billion respectively over the same period.
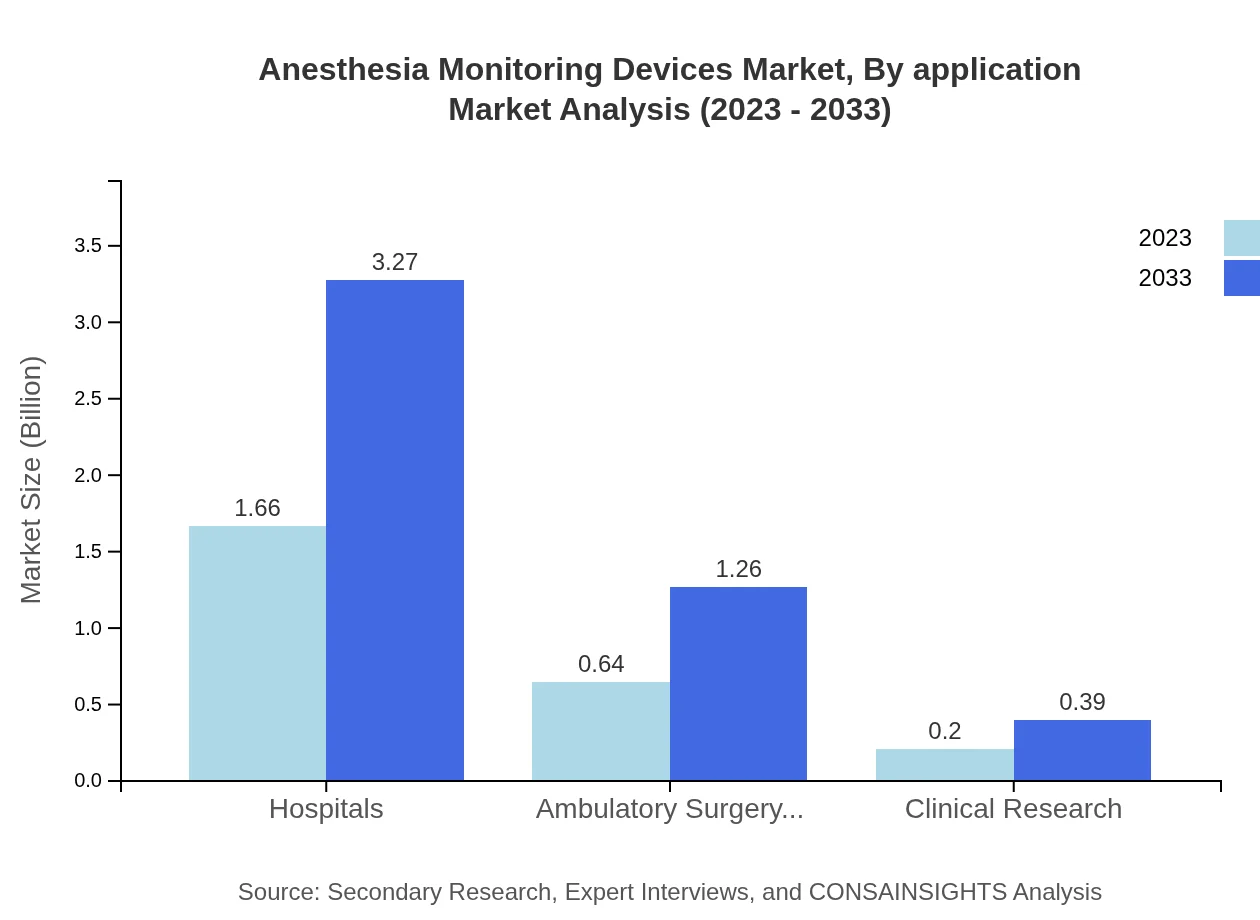
Anesthesia Monitoring Devices Market Analysis By Application
The applications of Anesthesia Monitoring Devices include various surgical procedures that utilize anesthesia. Anesthesiologists play a critical role and account for a market analysis size of USD 1.66 billion in 2023, expanding to USD 3.27 billion by 2033, maintaining a share of 66.55%. Surgeons constitute another substantial segment, with their total market size anticipated to reach USD 1.26 billion. Other healthcare providers occupy a smaller market segment, with projected sizes rising from USD 0.20 billion to USD 0.39 billion.
Anesthesia Monitoring Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Anesthesia Monitoring Devices Industry
Medtronic :
Medtronic is a global leader in medical technology, providing a broad range of products and solutions enhancing patient outcomes in anesthesia monitoring and management.Philips Healthcare:
Philips Healthcare innovates with advanced monitoring systems and technologies, emphasizing patient safety and efficacy in diverse healthcare settings.GE Healthcare:
GE Healthcare offers cutting-edge anesthesia monitoring devices that integrate intelligent imaging and analytics to improve patient safety and care workflows.Edwards Lifesciences:
Edwards Lifesciences specializes in heart valve products and critical care monitoring systems, profoundly impacting anesthesia monitoring practices.We're grateful to work with incredible clients.









FAQs
What is the market size of anesthesia Monitoring Devices?
The anesthesia monitoring devices market is valued at $2.5 billion in 2023, with an impressive CAGR of 6.8%. By 2033, the market is projected to grow significantly, indicating robust expansion in this vital healthcare sector.
What are the key market players or companies in this anesthesia Monitoring Devices industry?
The anesthesia monitoring devices market features key players including GE Healthcare, Philips, Mindray, Siemens Healthineers, and Drägerwerk. These companies are pivotal due to their innovative technologies and comprehensive product lines which dominate market share.
What are the primary factors driving the growth in the anesthesia Monitoring Devices industry?
Key factors driving growth include the increasing surgical procedures globally, advancements in monitoring technology, and the rising elderly population requiring surgical interventions. Enhanced patient safety protocols and investments in healthcare infrastructure also play crucial roles.
Which region is the fastest Growing in the anesthesia Monitoring Devices?
North America is the fastest-growing region in the anesthesia monitoring devices market, with a projected growth from $0.86 billion in 2023 to $1.69 billion by 2033, driven by high healthcare expenditure and technological advancements.
Does ConsaInsights provide customized market report data for the anesthesia Monitoring Devices industry?
Yes, ConsaInsights offers customized market report data tailored to the anesthesia monitoring devices industry. Clients can request specific insights, analyses, and forecasts to meet their unique business needs.
What deliverables can I expect from this anesthesia Monitoring Devices market research project?
Deliverables from the anesthesia monitoring devices market research project include detailed market analysis, size and segmentation data, competitive landscape insights, regional forecasts, and comprehensive trend analyses to support strategic decision-making.
What are the market trends of anesthesia Monitoring Devices?
Current trends in the anesthesia monitoring devices market include increased adoption of multi-parameter monitors, the shift towards digital monitoring technologies, and a focus on remote monitoring capabilities, which enhance patient safety and operational efficiency.