Anthrax Vaccine Market Report
Published Date: 31 January 2026 | Report Code: anthrax-vaccine
Anthrax Vaccine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Anthrax Vaccine market from 2023 to 2033, highlighting market size, growth trends, regional insights, industry analysis, and key players within the sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
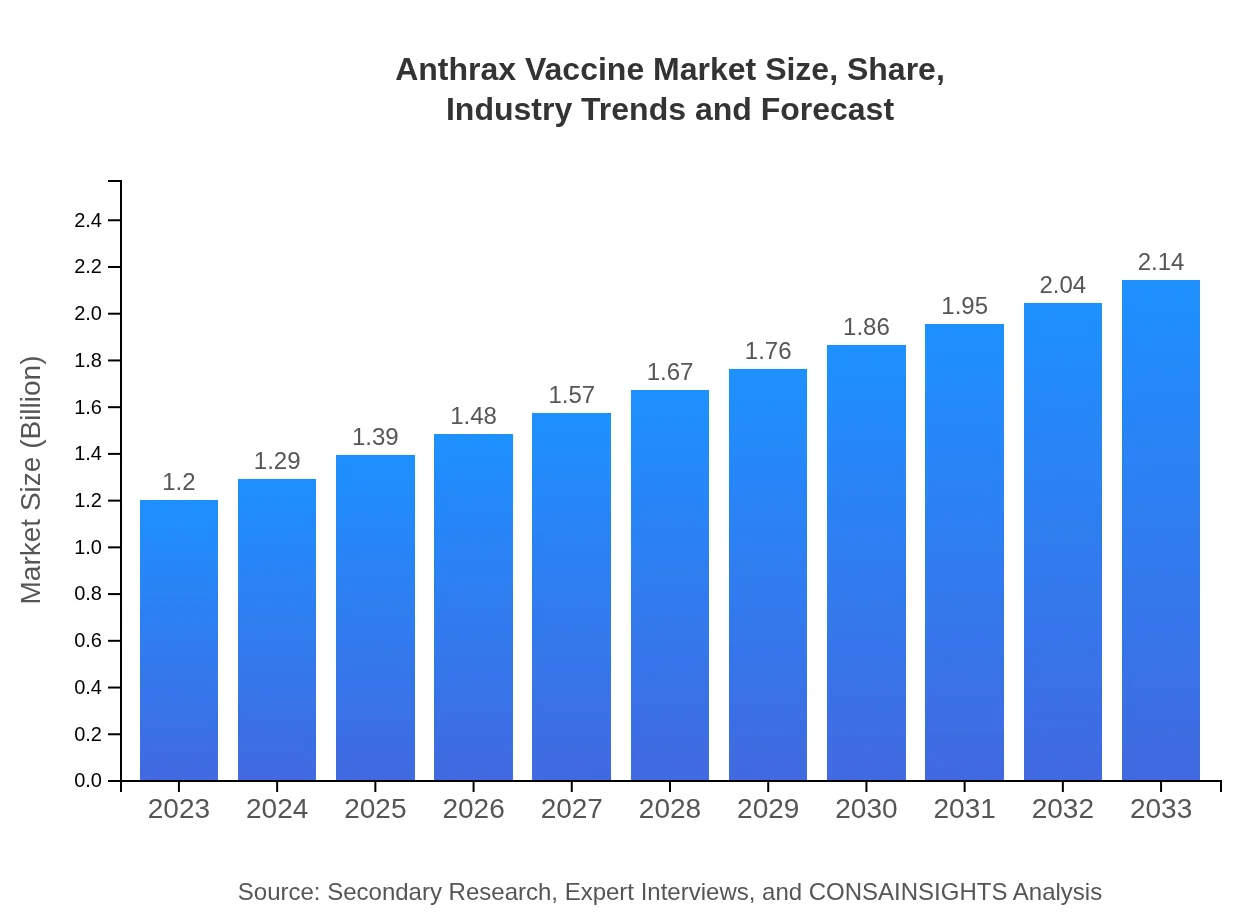
| 2023 Market Size | $1.20 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $2.14 Billion |
| Top Companies | Emergent BioSolutions, Sanofi Pasteur, GlaxoSmithKline (GSK), Pfizer |
| Last Modified Date | 31 January 2026 |
Anthrax Vaccine Market Overview
Customize Anthrax Vaccine Market Report market research report
- ✔ Get in-depth analysis of Anthrax Vaccine market size, growth, and forecasts.
- ✔ Understand Anthrax Vaccine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Anthrax Vaccine
What is the Market Size & CAGR of Anthrax Vaccine market in 2033?
Anthrax Vaccine Industry Analysis
Anthrax Vaccine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Anthrax Vaccine Market Analysis Report by Region
Europe Anthrax Vaccine Market Report:
Europe's Anthrax Vaccine market is estimated at $0.34 billion in 2023, forecasted to reach $0.60 billion by 2033. Increased vigilance against biological threats and a strong regulatory framework support market growth.Asia Pacific Anthrax Vaccine Market Report:
In 2023, the Asia Pacific Anthrax Vaccine market is valued at approximately $0.23 billion, projected to grow to $0.41 billion by 2033. The growing awareness of zoonotic diseases and increasing healthcare expenditure drive this region's growth.North America Anthrax Vaccine Market Report:
North America, dominated by the U.S. market, is significant, valued at $0.45 billion in 2023, expected to reach $0.80 billion by 2033. The North American market benefits from advanced healthcare systems, government-funded vaccination initiatives, and robust R&D environments.South America Anthrax Vaccine Market Report:
The South American Anthrax Vaccine market is estimated at $0.07 billion in 2023, with a projected growth to $0.12 billion by 2033. Limited access to vaccines and healthcare infrastructure challenges present growth opportunities for investment.Middle East & Africa Anthrax Vaccine Market Report:
The Middle East and Africa region is projected to grow from $0.12 billion in 2023 to $0.21 billion by 2033. Demand is driven by increasing instances of outbreaks and government initiatives to bolster vaccination rates in high-risk populations.Tell us your focus area and get a customized research report.
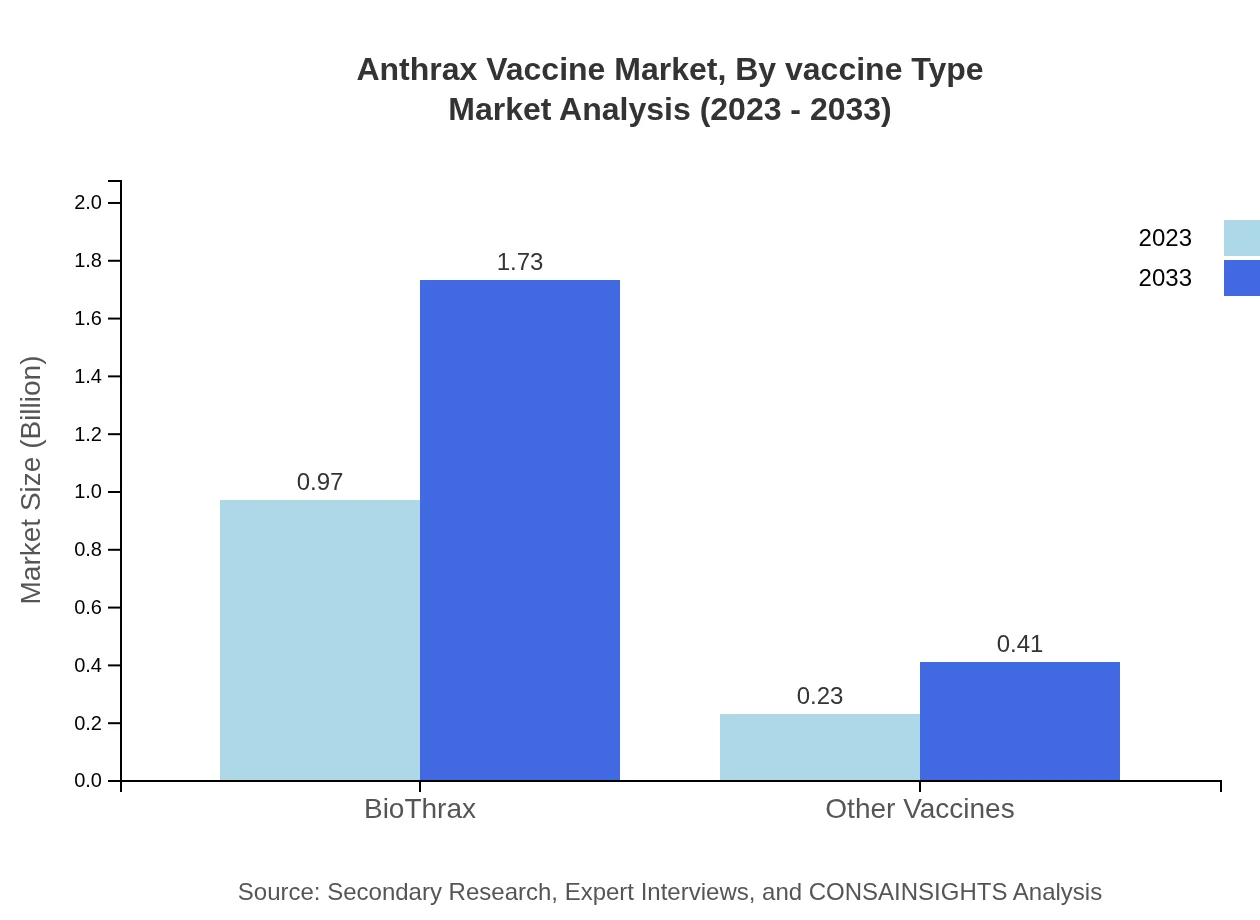
Anthrax Vaccine Market Analysis By Vaccine Type
In 2023, the segment of vaccines, notably BioThrax, leads the market with a size of $0.97 billion, projected to grow to $1.73 billion by 2033, maintaining an 80.95% market share. Other vaccines and investigational vaccines capture emerging opportunities for growth, especially in clinical and research applications.
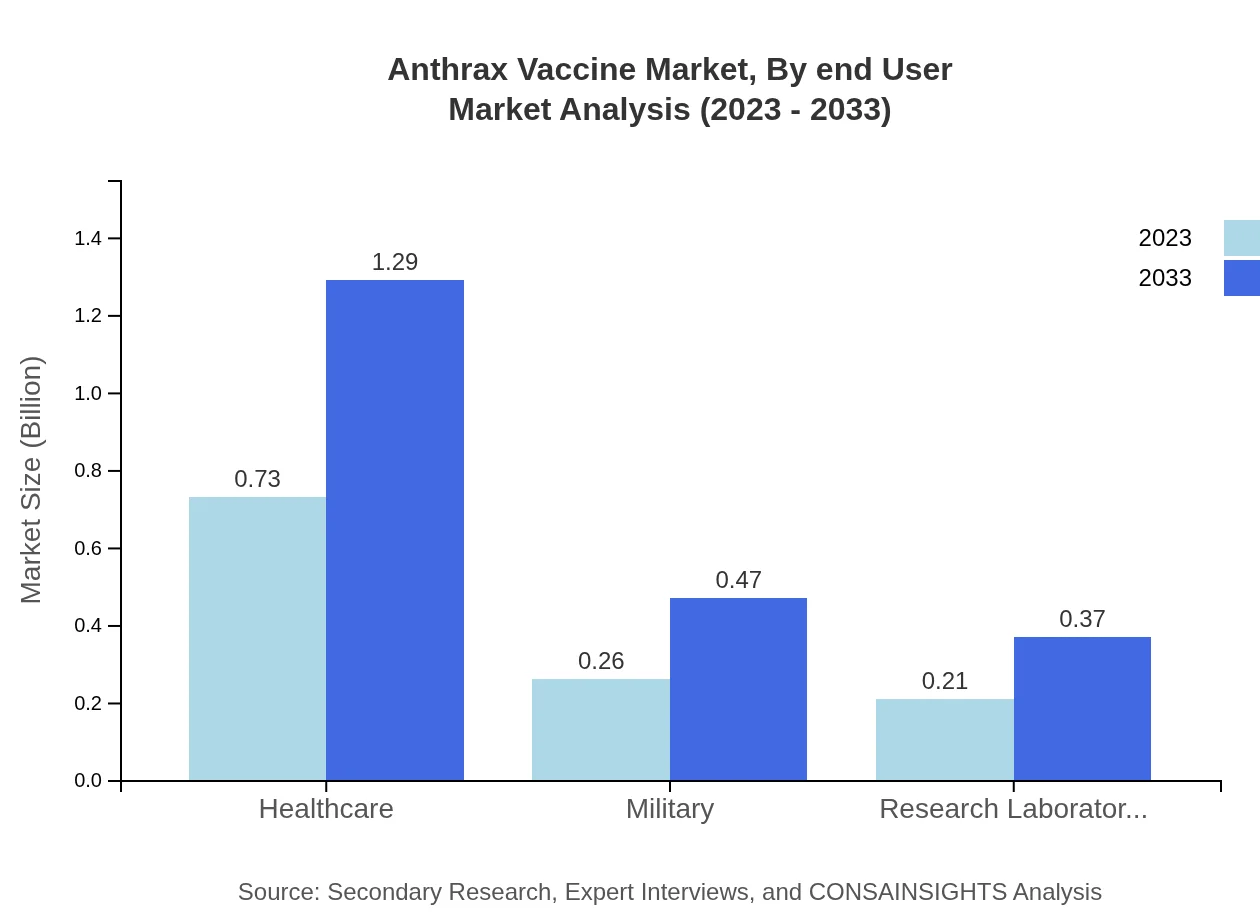
Anthrax Vaccine Market Analysis By End User
Segmentation by end-user in 2023 indicates healthcare dominates with a size of $0.73 billion and a share of 60.48%. Military applications follow closely at $0.26 billion (22% share), while research laboratories account for $0.21 billion at a 17.52% share. This distribution highlights the critical roles of public health safety and defense initiatives.
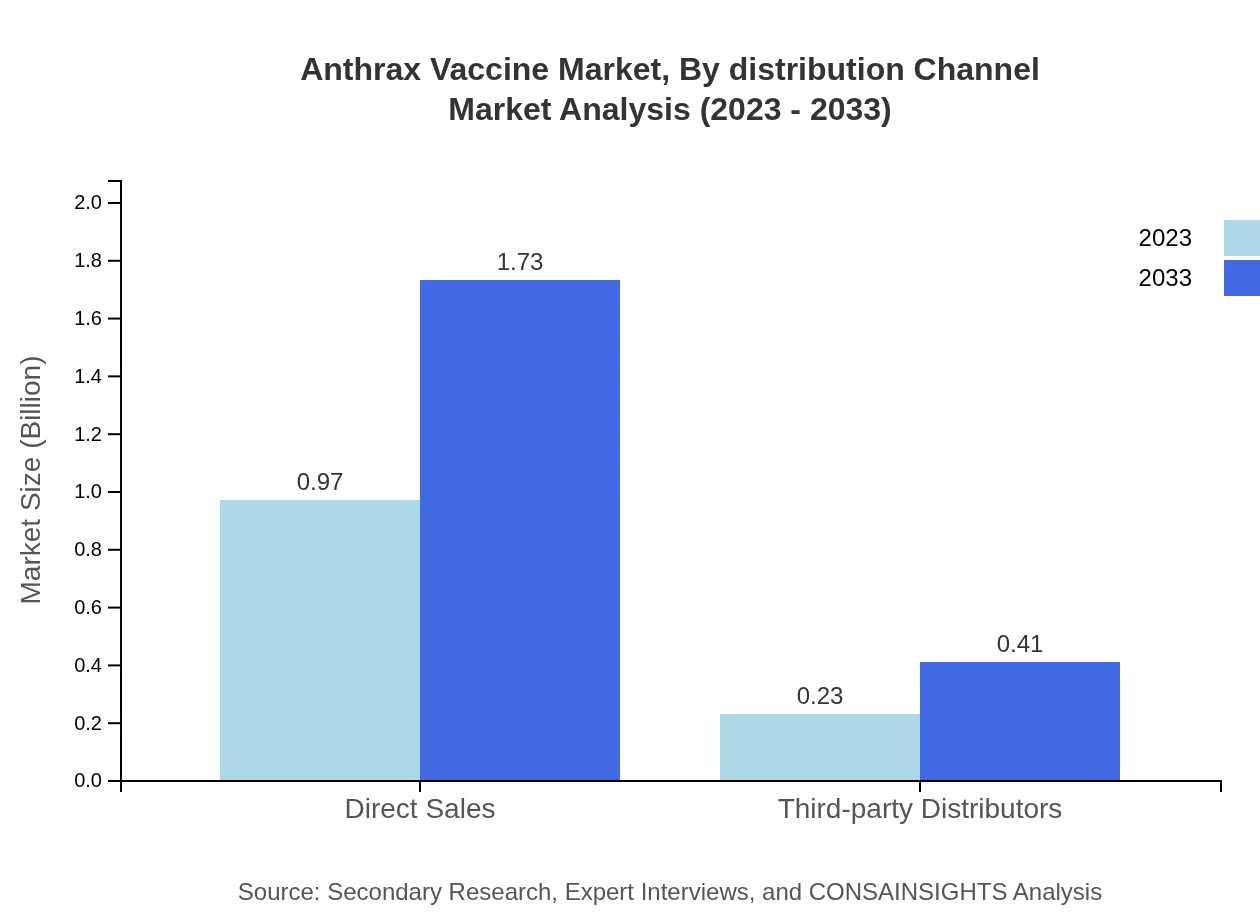
Anthrax Vaccine Market Analysis By Distribution Channel
Direct sales constitute the majority share, valued at $0.97 billion (80.95% share), with projected growth following the same trajectory. In comparison, third-party distributors represent a smaller segment at $0.23 billion (19.05% share), emphasized by significant relationships facilitating broader product access.
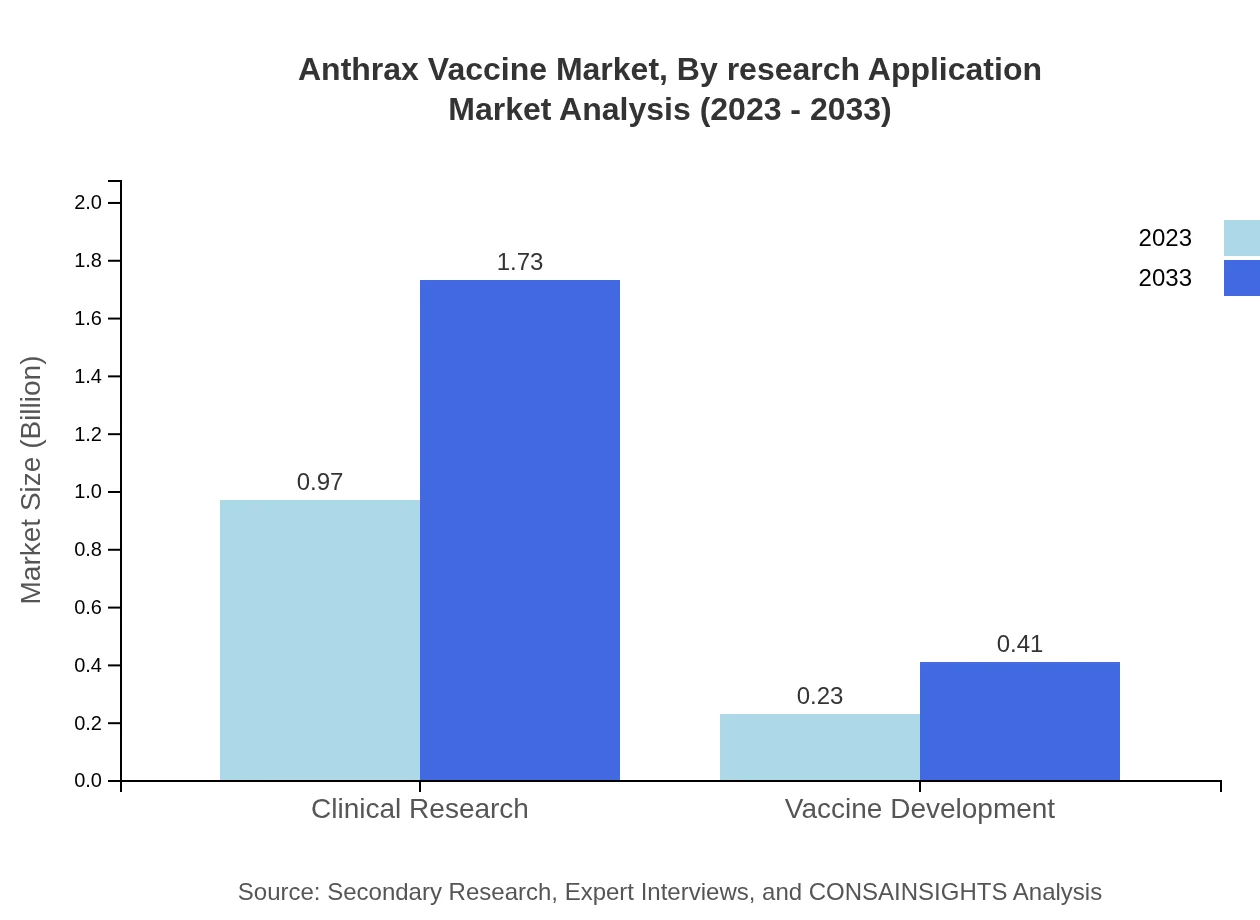
Anthrax Vaccine Market Analysis By Research Application
Clinical research holds a large market size at $0.97 billion (80.95% share) in 2023, indicating the importance of continuous product evaluation and innovation. Vaccine development applications also play a crucial role, estimated at $0.23 billion (19.05% share), signifying expanding research scopes.
Anthrax Vaccine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Anthrax Vaccine Industry
Emergent BioSolutions:
Emergent BioSolutions is the leading manufacturer of the BioThrax vaccine, playing a pivotal role in biopharmaceuticals focused on public health solutions. The company is noted for its innovative approaches and strong partnerships with government agencies.Sanofi Pasteur:
Sanofi Pasteur is a major player in vaccine development with a significant portfolio. The company focuses on research and provides vaccines across a broad range of infectious diseases, including anthrax.GlaxoSmithKline (GSK):
GSK is widely recognized for its global research and development framework in vaccines. The company's advancements in vaccine technology enhance the efficacy and safety of anthrax vaccines.Pfizer :
Pfizer's commitment to biopharmaceutical innovation extends to immunology and vaccine segments. With partnerships that facilitate vaccine research, Pfizer contributes to anthrax prevention strategies.We're grateful to work with incredible clients.









FAQs
What is the market size of anthrax Vaccine?
The global anthrax vaccine market is valued at approximately $1.2 billion in 2023, with a projected CAGR of 5.8% through 2033. This growth points towards an increasing focus on disease prevention and biosecurity, indicating a rising demand within this niche market.
What are the key market players or companies in the anthrax Vaccine industry?
Key players in the anthrax vaccine market include major pharmaceutical companies that specialize in vaccine development and biopharmaceutical production. Their continual investment in research and innovation drives competitive dynamics, influencing market stability and growth.
What are the primary factors driving the growth in the anthrax vaccine industry?
The growth of the anthrax vaccine market is primarily driven by increased biological threat awareness, rising government initiatives for vaccination programs, and the growing necessity for bio-defense mechanisms in healthcare and military settings.
Which region is the fastest Growing in the anthrax vaccine market?
The North American region is observing the fastest growth in the anthrax vaccine market, projected to expand from $0.45 billion in 2023 to $0.80 billion by 2033, propelled by strong healthcare regulations and heightened defense requirements.
Does Consainsights provide customized market report data for the anthrax vaccine industry?
Yes, Consainsights offers customized market report data tailored to the anthrax vaccine industry. Clients can request specific insights according to their unique needs, including regional analyses, competitor landscapes, and segment-focused reporting.
What deliverables can I expect from this anthrax vaccine market research project?
Deliverables from the anthrax vaccine market research project include comprehensive reports covering market size, growth forecasts, regional analyses, competitive landscapes, and key trends influencing the market over the projected period.
What are the market trends of anthrax vaccine?
Trends in the anthrax vaccine market indicate increasing investments in biopharmaceutical research, advancements in vaccine formulation technology, and a growing emphasis on partnerships between public and private sectors to enhance vaccine accessibility and distribution.