Anti Viral Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: anti-viral-therapeutics
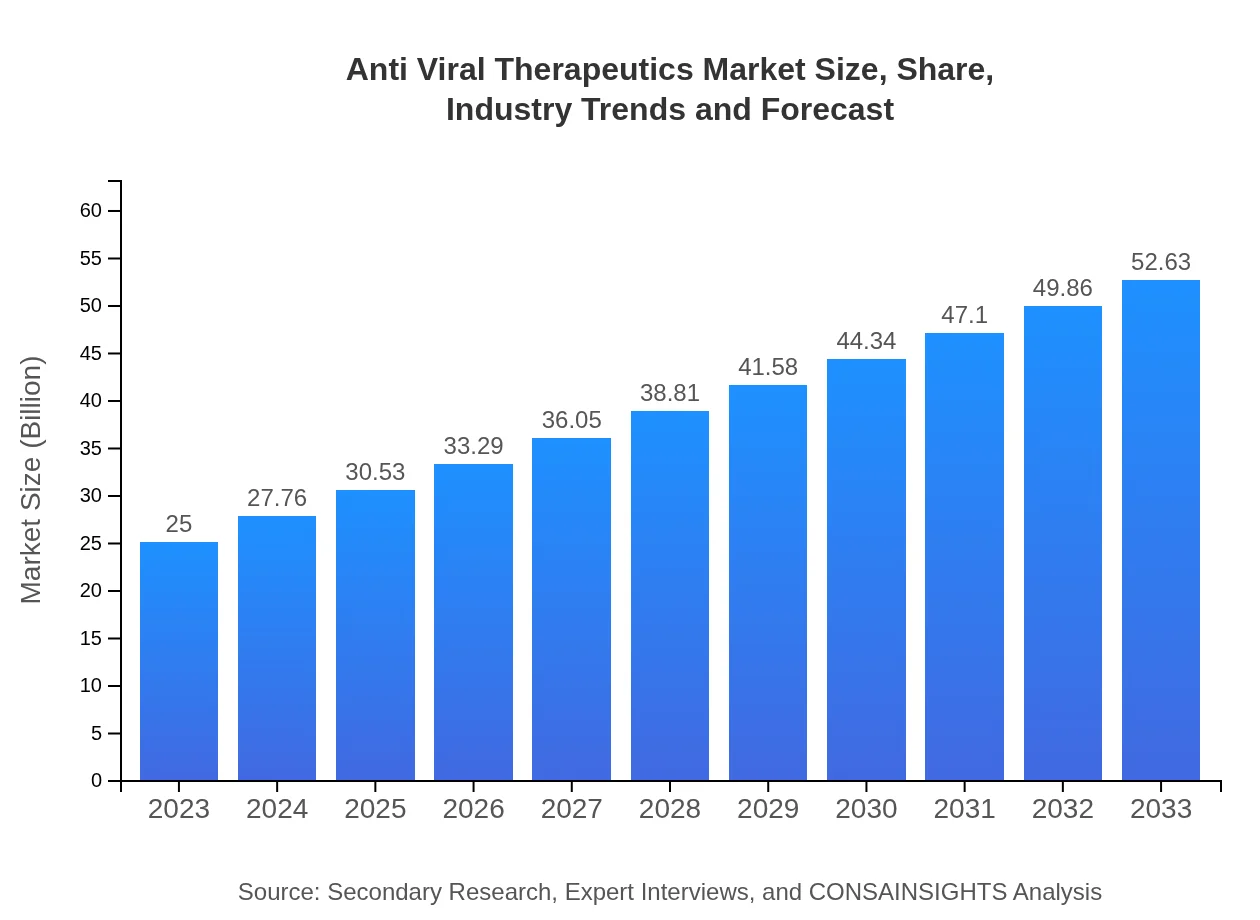
Anti Viral Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report presents a comprehensive analysis of the Anti Viral Therapeutics market for the forecast period from 2023 to 2033. It includes insights into market trends, size, growth rates, key segments, regional performances, and characteristics of leading players in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $25.00 Billion |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $52.63 Billion |
| Top Companies | Gilead Sciences, Inc., GlaxoSmithKline plc, AbbVie Inc., Merck & Co., Inc., Bristol-Myers Squibb Company |
| Last Modified Date | 31 January 2026 |
Anti Viral Therapeutics Market Overview
Customize Anti Viral Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Anti Viral Therapeutics market size, growth, and forecasts.
- ✔ Understand Anti Viral Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Anti Viral Therapeutics
What is the Market Size & CAGR of Anti Viral Therapeutics market in 2023?
Anti Viral Therapeutics Industry Analysis
Anti Viral Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Anti Viral Therapeutics Market Analysis Report by Region
Europe Anti Viral Therapeutics Market Report:
The European market is anticipated to grow from USD 8.00 billion in 2023 to USD 16.83 billion by 2033. This expansion is driven by increasing incidences of viral infections and advancements in therapeutics supported by robust regulatory frameworks and healthcare systems.Asia Pacific Anti Viral Therapeutics Market Report:
In the Asia Pacific region, the Anti Viral Therapeutics market was valued at USD 4.86 billion in 2023 and is expected to reach USD 10.23 billion by 2033. The growth can be attributed to increasing healthcare expenditure, rising awareness of viral diseases, and the rising population requiring antiviral treatments.North America Anti Viral Therapeutics Market Report:
North America, with a market size of USD 8.14 billion in 2023, is projected to reach USD 17.14 billion by 2033, supported by high R&D spending, a strong pharmaceutical industry, and widespread availability of antiviral treatments.South America Anti Viral Therapeutics Market Report:
The South American market was valued at USD 1.23 billion in 2023, projected to grow to USD 2.58 billion by 2033. Factors like improving healthcare infrastructure and increasing investments in healthcare are fostering market growth in the region.Middle East & Africa Anti Viral Therapeutics Market Report:
In the Middle East and Africa, the market was valued at USD 2.78 billion in 2023, with projections reaching USD 5.85 billion by 2033. Growth is supported by improved access to healthcare and rising awareness of antiviral therapies.Tell us your focus area and get a customized research report.
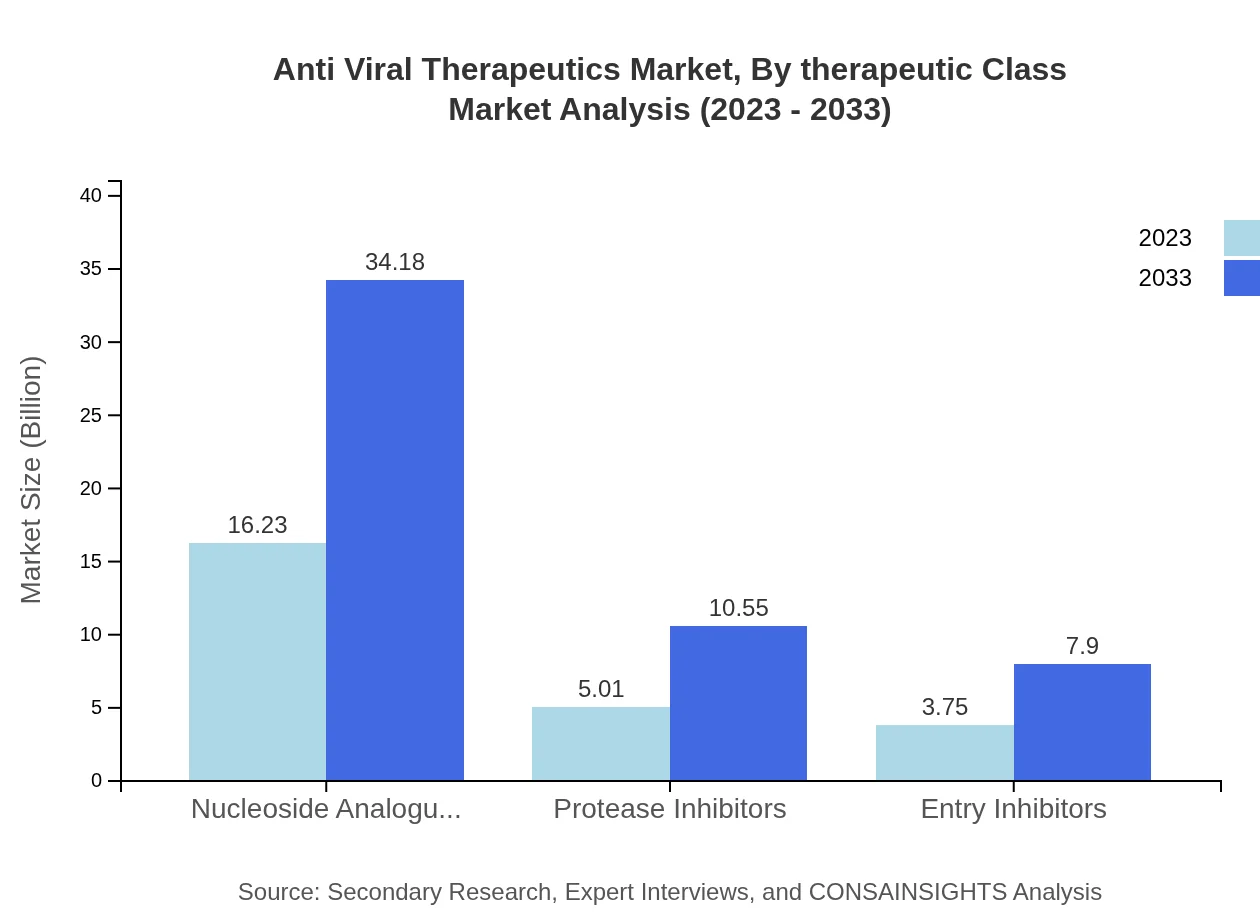
Anti Viral Therapeutics Market Analysis By Therapeutic Class
The therapeutic class segment of the Anti Viral Therapeutics market is dominated by Nucleoside Analogues, which celebrated a market size of USD 16.23 billion in 2023, projected to increase to USD 34.18 billion in 2033, representing 64.94% of the global market share in both years. This class remains vital due to its efficacy against various viral infections.
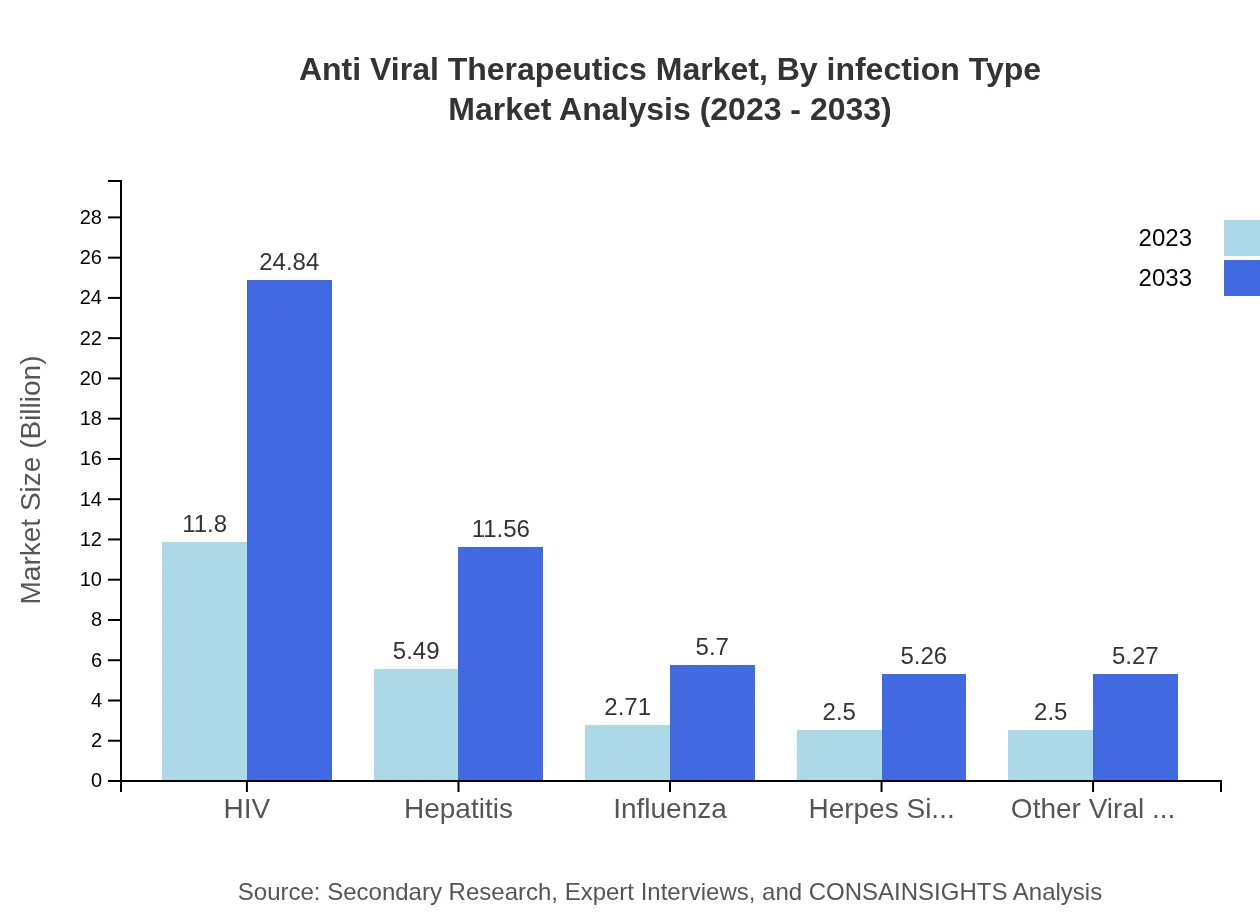
Anti Viral Therapeutics Market Analysis By Infection Type
The HIV segment continues to lead the market with a size of USD 11.80 billion in 2023 and projected growth to USD 24.84 billion by 2033, maintaining a 47.2% market share. The Hepatitis sector comes next, valued at USD 5.49 billion in 2023 and reaching USD 11.56 billion by 2033.
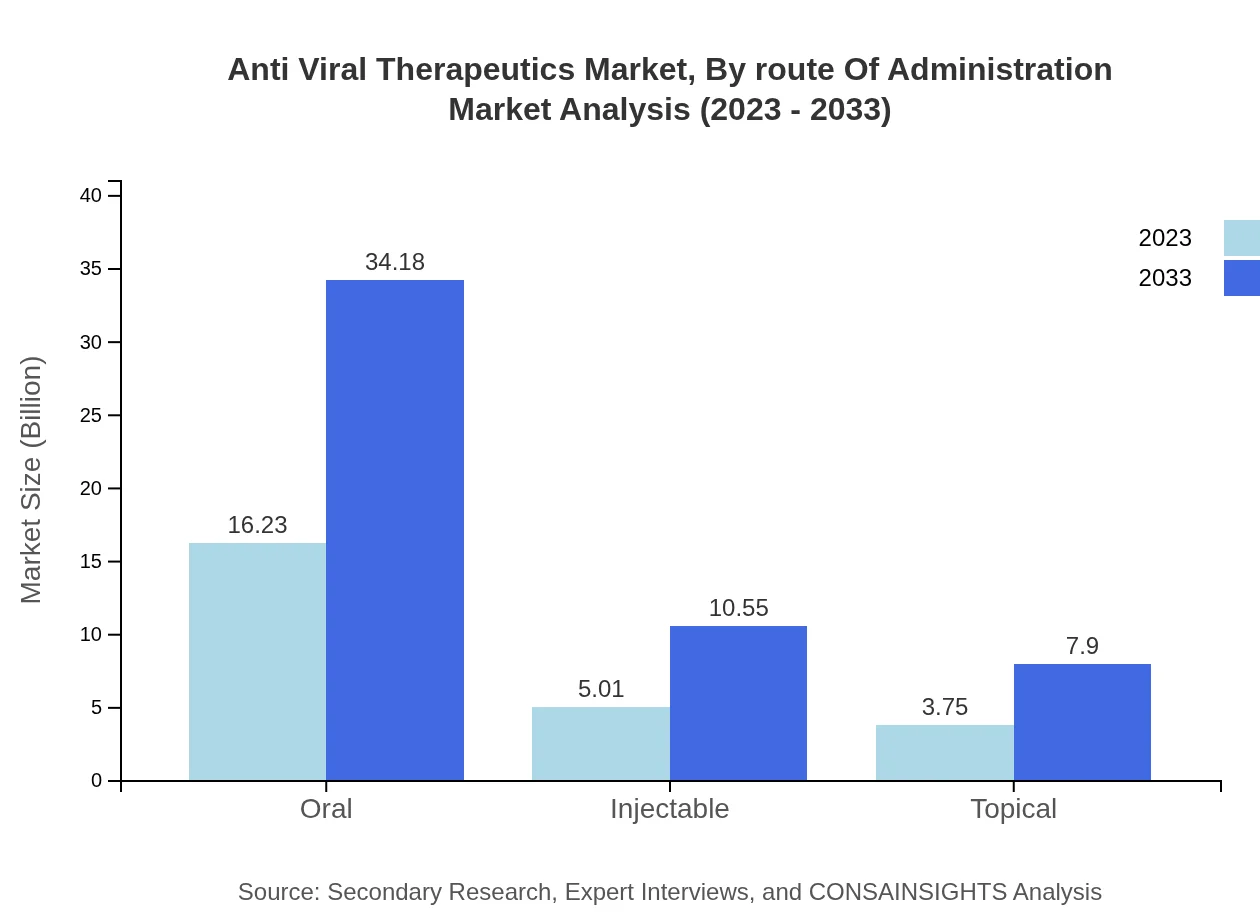
Anti Viral Therapeutics Market Analysis By Route Of Administration
In terms of routes of administration, oral medications held a dominant position, with a market size of USD 16.23 billion in 2023 and projected to reach USD 34.18 billion by 2033, reflecting a steady preference for non-invasive treatment options.
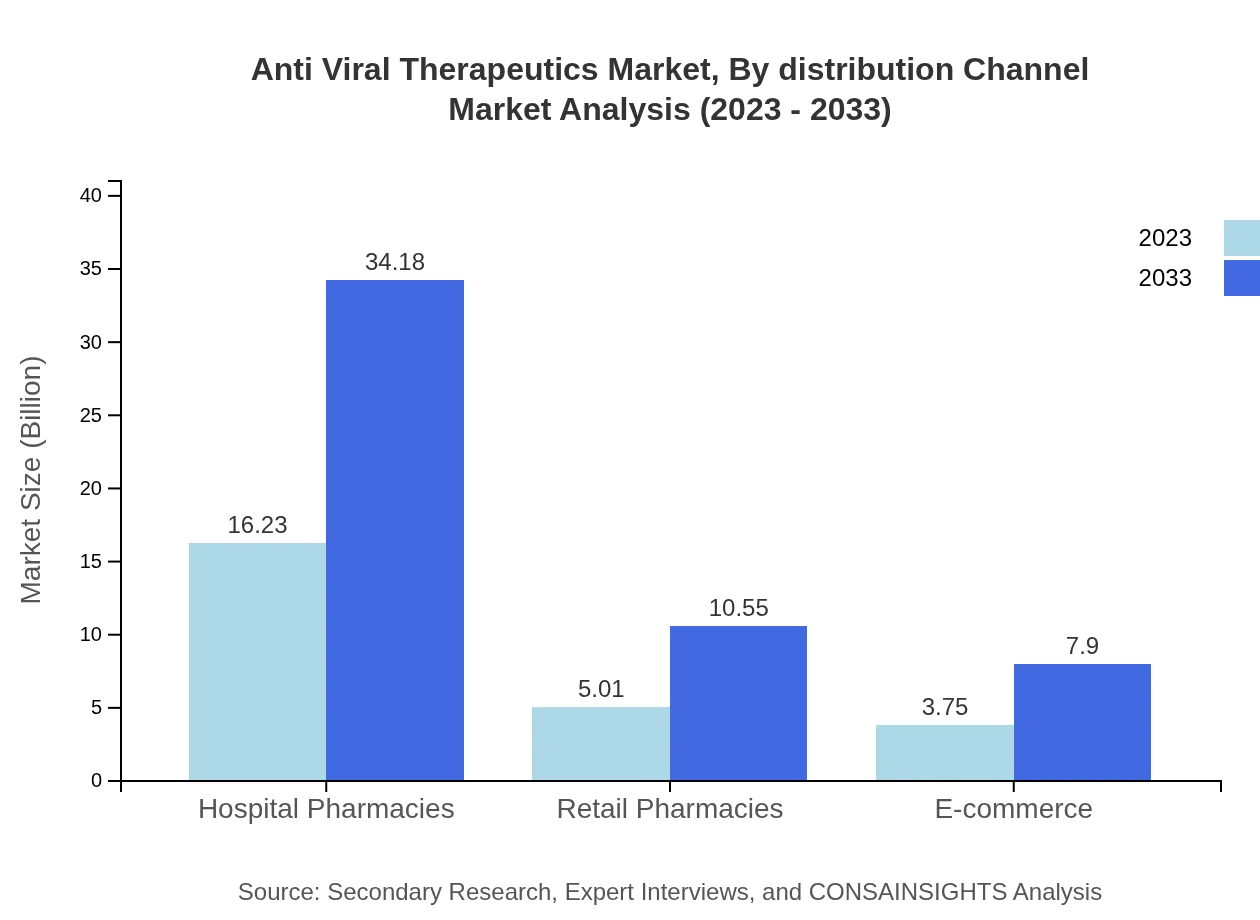
Anti Viral Therapeutics Market Analysis By Distribution Channel
Hospital pharmacies accounted for a projected market size of USD 16.23 billion in 2023, anticipated to grow to USD 34.18 billion by 2033, exhibiting the significance of hospital settings in administering antiviral medications.
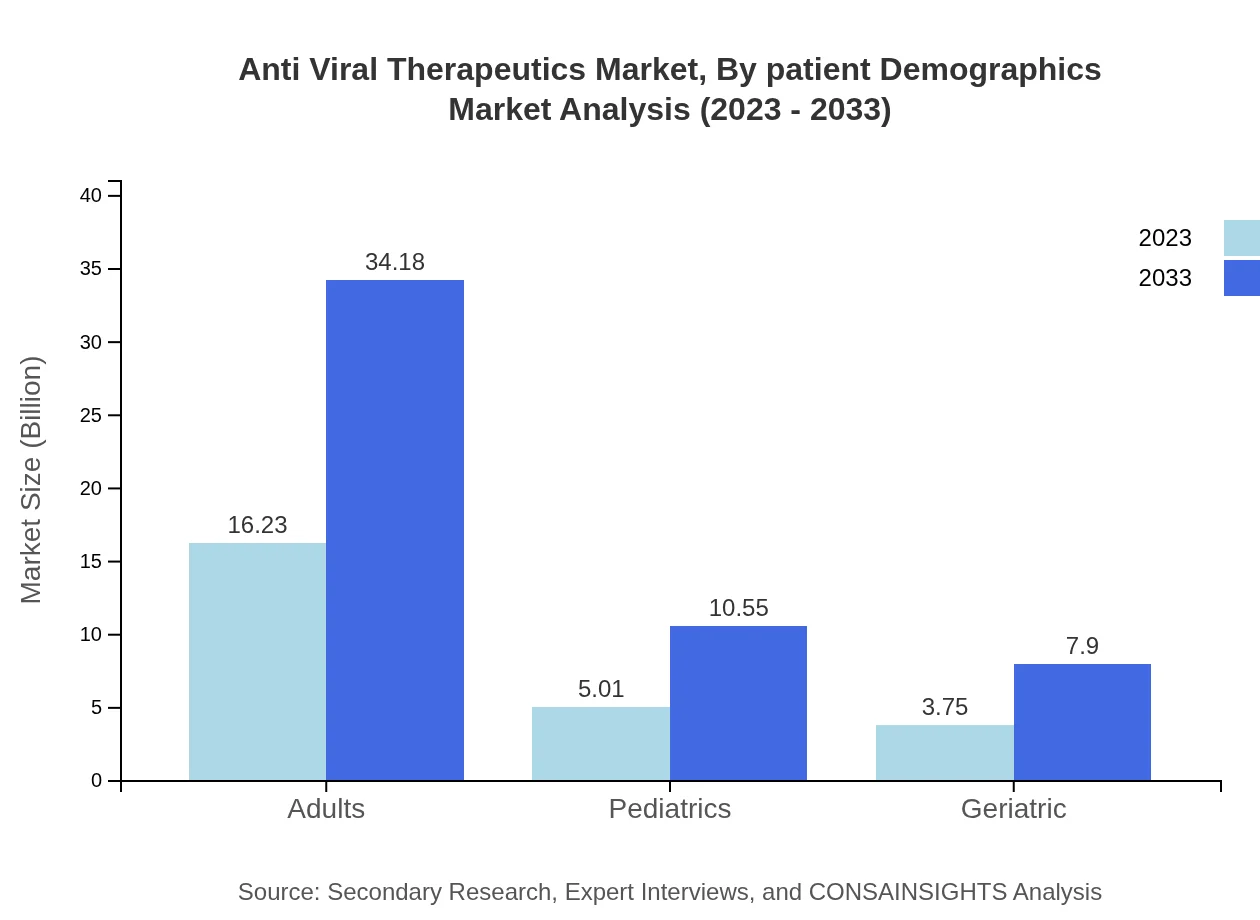
Anti Viral Therapeutics Market Analysis By Patient Demographics
The adult demographic remains the largest consumer category of antiviral treatments, holding a market size of USD 16.23 billion in 2023 and expected to grow to USD 34.18 billion by 2033, underscoring the need for effective treatments in the adult population.
Anti Viral Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Anti Viral Therapeutics Industry
Gilead Sciences, Inc.:
A leader in antiviral therapeutics, Gilead has been pivotal in developing breakthrough treatments for HIV and Hepatitis, significantly influencing patient outcomes globally.GlaxoSmithKline plc:
GlaxoSmithKline has a robust portfolio in the antiviral market, focusing on innovative treatments for influenza and HIV, cementing its position as a market leader.AbbVie Inc.:
AbbVie's advancements in the antiviral space, especially concerning Hepatitis C, have transformed standard care practices and currently leads in several antiviral drug classes.Merck & Co., Inc.:
Merck has established itself in the antiviral market with a strong focus on HIV medications, and its notable contribution to COVID-19 antivirals has further enhanced its reputation.Bristol-Myers Squibb Company:
Bristol-Myers Squibb's commitment to research and comprehensive approaches to antiviral therapies makes it a key player in managing viral diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of anti Viral Therapeutics?
The anti-viral therapeutics market is estimated to reach approximately $25 billion by 2033, growing at a CAGR of 7.5%. This growth reflects increasing demand for viral infection treatments and advancements in drug development.
What are the key market players or companies in this anti Viral Therapeutics industry?
Key players in the anti-viral therapeutics market include major pharmaceutical companies such as Gilead Sciences, GlaxoSmithKline, and AbbVie, which are at the forefront of developing innovative antiviral drugs and therapies.
What are the primary factors driving the growth in the anti Viral Therapeutics industry?
Growth in the anti-viral therapeutics industry is driven by the rising prevalence of viral infections, advancements in biotechnology, accelerated drug approvals, and the increasing demand for effective treatment options worldwide.
Which region is the fastest Growing in the anti Viral Therapeutics?
North America is the fastest-growing region in the anti-viral therapeutics market, projected to expand from $8.14 billion in 2023 to $17.14 billion by 2033.
Does ConsaInsights provide customized market report data for the anti Viral Therapeutics industry?
Yes, ConsaInsights offers customized market reports tailored specifically to client needs, enabling stakeholders to access detailed insights and analytics for the anti-viral therapeutics industry.
What deliverables can I expect from this anti Viral Therapeutics market research project?
Clients can expect comprehensive reports including market analysis, segmentation data, growth forecasts, competitive landscape assessments, and insights into emerging trends in the anti-viral therapeutics market.
What are the market trends of anti Viral Therapeutics?
Current trends in the anti-viral therapeutics market include the increasing focus on personalized medicine, adoption of combination therapies, and advancements in drug delivery systems, emphasizing improved efficacy and patient adherence.