Antibody Production Market Report
Published Date: 31 January 2026 | Report Code: antibody-production
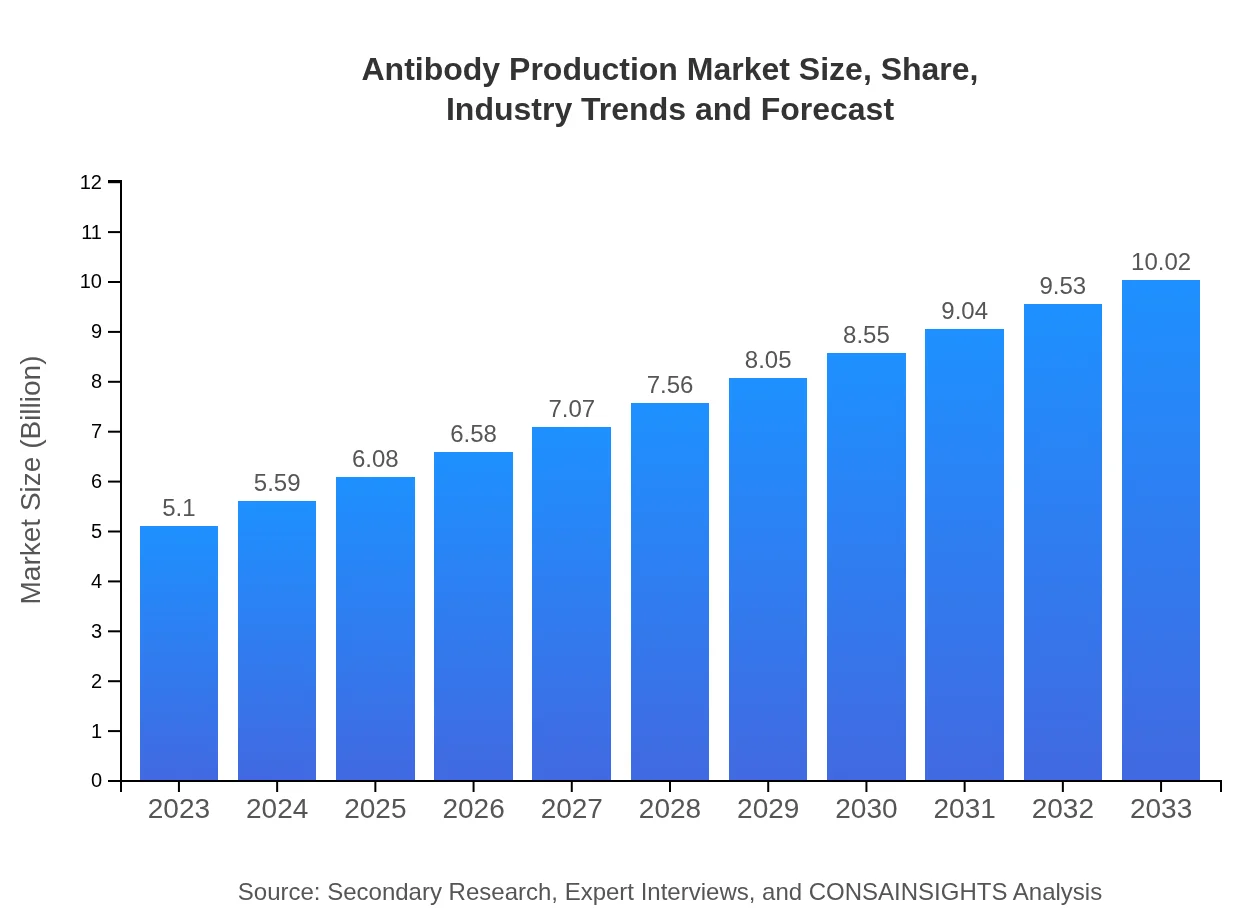
Antibody Production Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Antibody Production market, covering key insights, market size, trends, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.10 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.02 Billion |
| Top Companies | Roche, AbbVie, Amgen, Johnson & Johnson |
| Last Modified Date | 31 January 2026 |
Antibody Production Market Overview
Customize Antibody Production Market Report market research report
- ✔ Get in-depth analysis of Antibody Production market size, growth, and forecasts.
- ✔ Understand Antibody Production's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Antibody Production
What is the Market Size & CAGR of Antibody Production market in 2023?
Antibody Production Industry Analysis
Antibody Production Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Antibody Production Market Analysis Report by Region
Europe Antibody Production Market Report:
The European Antibody Production market is projected to expand from $1.26 billion in 2023 to $2.48 billion by 2033. The region is characterized by a strong focus on biopharmaceutical research, with numerous collaborations between academic institutions and industry leaders. Regulatory frameworks are supportive of innovation, aiding in accelerating antibody development and approval processes.Asia Pacific Antibody Production Market Report:
The Asia Pacific region holds considerable potential for growth, with the market expected to rise from $0.98 billion in 2023 to $1.92 billion in 2033. Rapid economic growth, increased R&D investments, and a surging demand for high-quality biologics are key factors contributing to this growth. Additionally, several countries in this region are enhancing their manufacturing capabilities, supporting local production of therapeutic antibodies.North America Antibody Production Market Report:
With a robust market size growing from $1.92 billion in 2023 to approximately $3.77 billion by 2033, North America stands as the largest regional market for antibody production. The USA leads due to its dominance in biopharmaceutical research and development, coupled with significant investments in healthcare and biotechnology. The presence of major pharmaceutical companies and stringent regulatory standards drive advancements in quality and efficacy.South America Antibody Production Market Report:
The South American Antibody Production market, projected to grow from $0.28 billion in 2023 to $0.55 billion by 2033, is benefiting from an expanding healthcare infrastructure and rising demand for antibody-based therapies. Although still in the nascent stages compared to other regions, government initiatives to boost biotechnology can propel growth in the upcoming years.Middle East & Africa Antibody Production Market Report:
The Middle East and Africa market is anticipated to grow from $0.67 billion in 2023 to $1.31 billion by 2033. Increased investments in healthcare, coupled with a rising incidence of diseases such as cancer, are likely to drive market growth. However, regional disparities in healthcare access and infrastructure may pose challenges to market expansion.Tell us your focus area and get a customized research report.
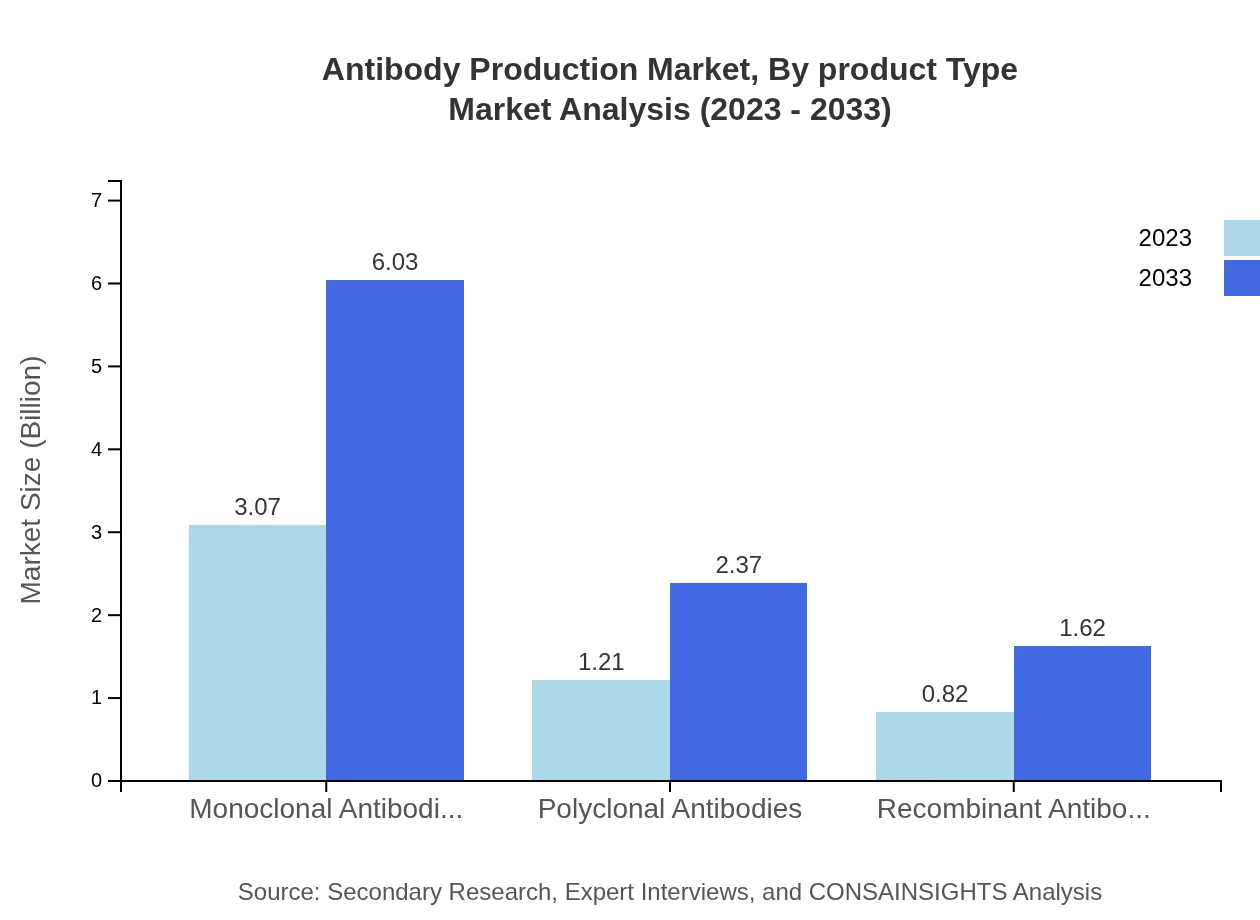
Antibody Production Market Analysis By Product Type
The market for antibody production by product type is dominated by monoclonal antibodies, accounting for approximately $3.07 billion in 2023, projected to rise to $6.03 billion in 2033. This segment's growth is attributed to rising therapeutic applications and enhanced product efficacy. Polyclonal antibodies and recombinant antibodies also contribute, expected to grow significantly from $1.21 billion to $2.37 billion and $0.82 billion to $1.62 billion respectively, driven by expanding diagnostic and therapeutic applications.
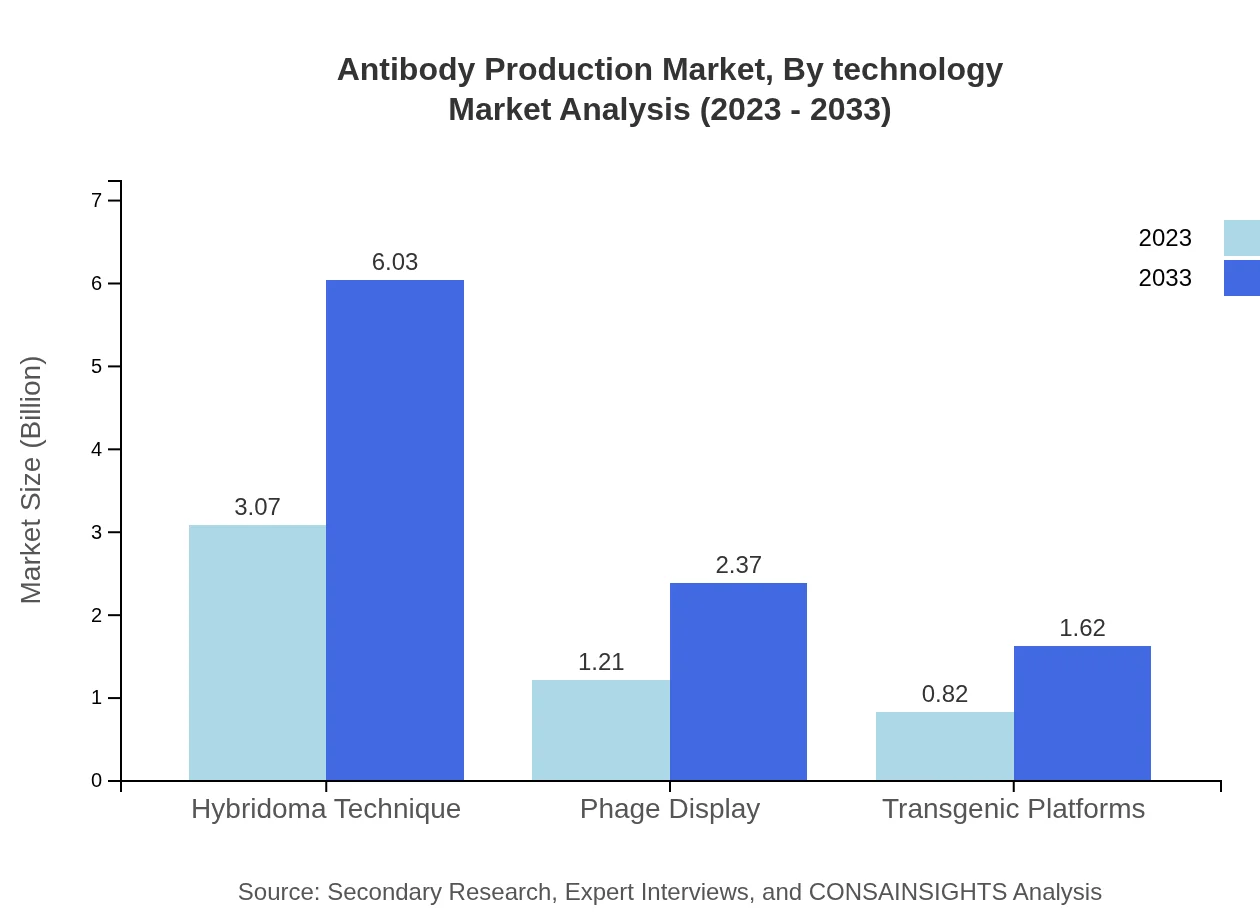
Antibody Production Market Analysis By Technology
In the technology segment, hybridoma techniques dominate with a market size of $3.07 billion in 2023, emphasizing its vital role in monoclonal antibody production. Phage display technology accounts for approximately $1.21 billion and is projected to grow similarly. Transgenic platforms are gradually gaining acceptance in the market, forecasted to expand from $0.82 billion to $1.62 billion due to their capability to produce high-yield antibodies.
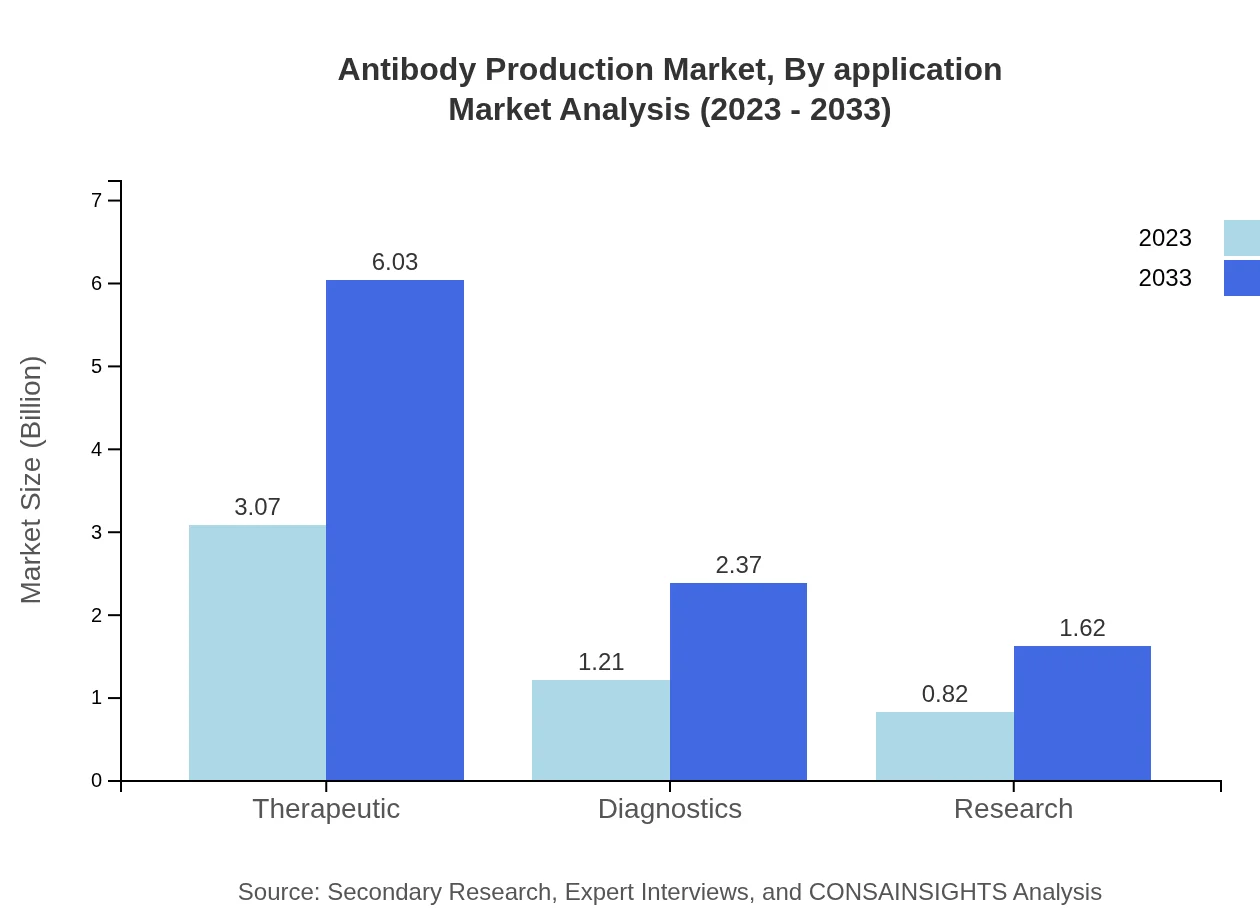
Antibody Production Market Analysis By Application
The application of antibodies spans therapeutics and diagnostics, with therapeutics commanding a significant share. Therapeutic applications account for approximately $3.07 billion in 2023, expected to rise to $6.03 billion. Diagnostics applications also showcase growth potential, moving from $1.21 billion to $2.37 billion in the same timeframe, highlighting an increasing demand for rapid testing and personalized medicine solutions.
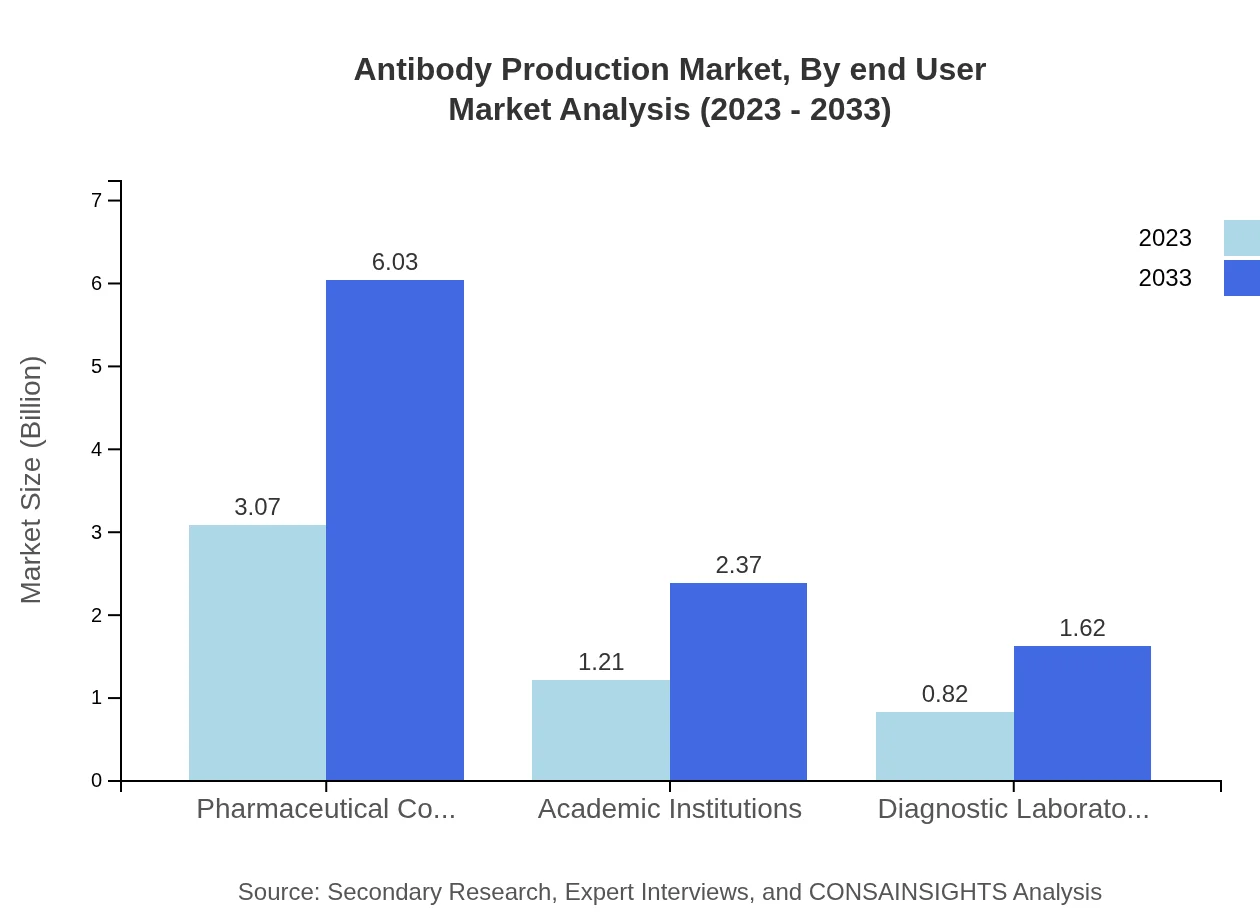
Antibody Production Market Analysis By End User
End-users in the antibody production market include pharmaceutical companies, academic institutions, and diagnostic laboratories. Pharmaceutical companies currently dominate the market with $3.07 billion, representing a 60.18% market share in 2023. Academic institutions and diagnostic labs also play crucial roles in research and development, with shares of 23.69% and 16.13% respectively, highlighting the holistic approach needed for innovation in the antibody sector.
Antibody Production Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Antibody Production Industry
Roche:
A leader in biotechnology and diagnostics, Roche specializes in monoclonal antibody therapies and diagnostics, driving significant innovations in antibody production.AbbVie:
AbbVie is known for its successful line of monoclonal antibodies, catering to several therapeutic areas, including immunology and oncology.Amgen:
Amgen focuses on developing advanced antibody-based therapies, particularly in oncology and chronic disease management, making substantial contributions to the industry.Johnson & Johnson:
With a diversified portfolio that includes therapeutic antibodies, Johnson & Johnson remains a significant player, investing heavily in R&D.We're grateful to work with incredible clients.









FAQs
What is the market size of antibody production?
The global antibody production market is valued at approximately $5.1 billion, with a projected compound annual growth rate (CAGR) of 6.8% from 2023 to 2033, indicating significant growth and demand in the upcoming years.
What are the key market players or companies in the antibody production industry?
Key market players in the antibody production industry include leading pharmaceutical companies and biotechnology firms such as Amgen, Genentech, and AbbVie, which are pivotal in enhancing antibody production techniques and applications.
What are the primary factors driving the growth in the antibody production industry?
Growth in the antibody production industry is primarily driven by the increasing prevalence of chronic diseases, advancements in biotechnology, and the rising demand for personalized medicine, which requires efficient antibody production systems.
Which region is the fastest Growing in the antibody production market?
The fastest-growing region in the antibody production market is North America, projected to expand from $1.92 billion in 2023 to $3.77 billion by 2033, fueled by robust research initiatives and innovative healthcare solutions.
Does ConsaInsights provide customized market report data for the antibody production industry?
Yes, ConsaInsights specializes in providing customized market report data tailored to the specific needs and goals of clients within the antibody production industry, ensuring relevant insights and strategic recommendations.
What deliverables can I expect from this antibody production market research project?
From the antibody production market research project, you can expect detailed reports covering market size, growth projections, competitive landscape analysis, regional insights, and segment-specific data for informed decision-making.
What are the market trends of antibody production?
Current trends in antibody production include a shift towards monoclonal antibodies, increased adoption of phage display technology, and a focus on transgenic platforms, all contributing to enhanced therapeutic and diagnostic applications.