Antiinfective Endotracheal Tube Market Report
Published Date: 31 January 2026 | Report Code: antiinfective-endotracheal-tube
Antiinfective Endotracheal Tube Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the anti-infective endotracheal tube market, including key insights, segmentation, and forecasting from 2023 to 2033. It aims to give stakeholders an understanding of market dynamics, trends, and future growth potential.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
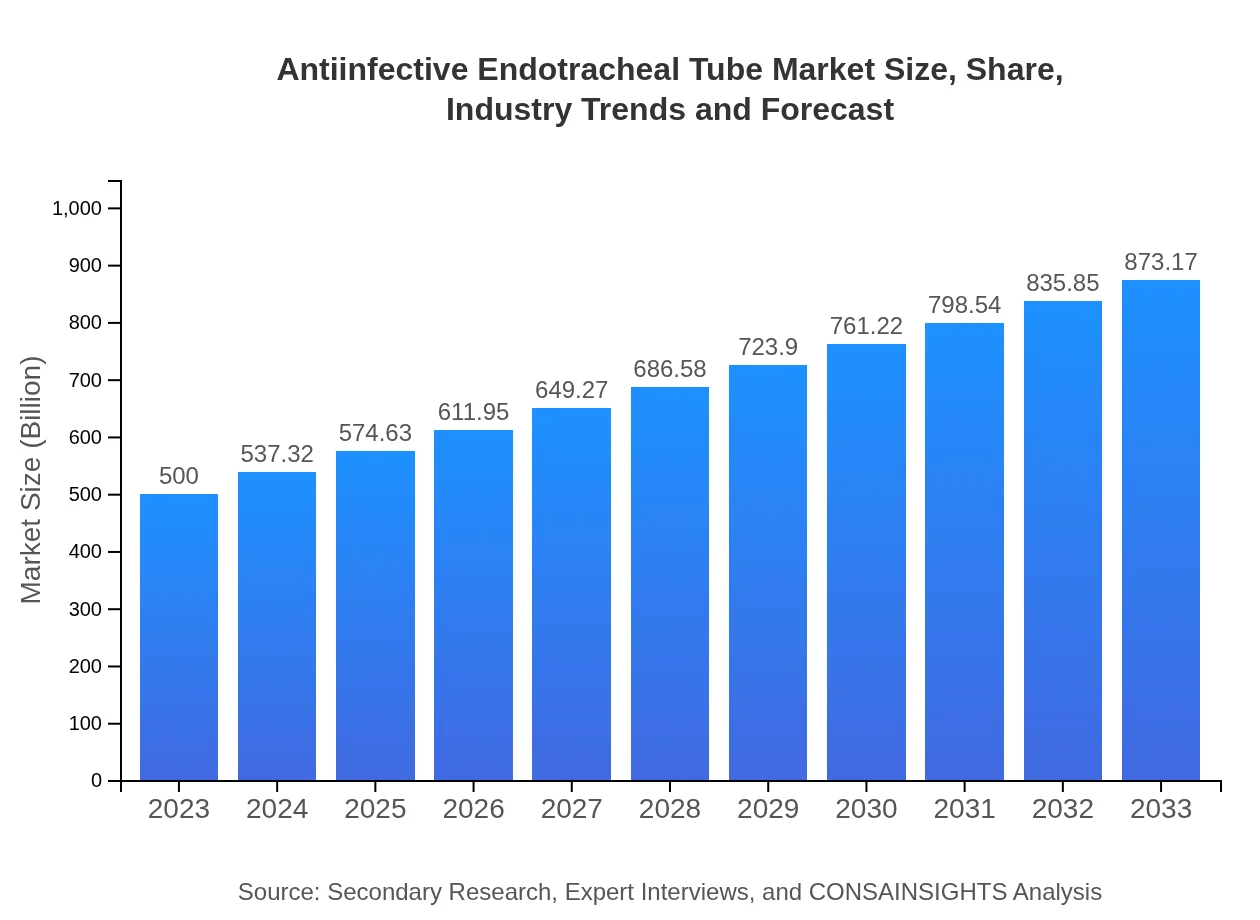
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.6% |
| 2033 Market Size | $873.17 Million |
| Top Companies | Medtronic , Boston Scientific, Teleflex, Smiths Medical, Hollister Incorporated |
| Last Modified Date | 31 January 2026 |
Anti-infective Endotracheal Tube Market Overview
Customize Antiinfective Endotracheal Tube Market Report market research report
- ✔ Get in-depth analysis of Antiinfective Endotracheal Tube market size, growth, and forecasts.
- ✔ Understand Antiinfective Endotracheal Tube's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Antiinfective Endotracheal Tube
What is the Market Size & CAGR of the Anti-infective Endotracheal Tube market in 2023?
Anti-infective Endotracheal Tube Industry Analysis
Anti-infective Endotracheal Tube Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Anti-infective Endotracheal Tube Market Analysis Report by Region
Europe Antiinfective Endotracheal Tube Market Report:
In Europe, the market for anti-infective endotracheal tubes is estimated to grow from USD 181.70 million in 2023 to USD 317.31 million by 2033. Regulatory frameworks emphasizing patient safety and rising investments in healthcare technologies are key drivers in this region. Increasing focus on reducing hospital-acquired infections is further propelling the demand.Asia Pacific Antiinfective Endotracheal Tube Market Report:
In the Asia Pacific region, the anti-infective endotracheal tube market is projected to grow from USD 80.45 million in 2023 to USD 140.49 million in 2033. Rapidly expanding healthcare infrastructure, coupled with rising instances of respiratory diseases, contributes to this growth. Growing awareness surrounding infection control in healthcare settings is also reinforcing demand for anti-infective devices.North America Antiinfective Endotracheal Tube Market Report:
North America remains a dominant market, valued at USD 164.35 million in 2023, with forecasts of USD 287.01 million by 2033. This region showcases advanced healthcare infrastructure and high rates of surgical procedures, which foster an environment for rapid adoption of anti-infective endotracheal tubes. Continuous innovations are also driving growth.South America Antiinfective Endotracheal Tube Market Report:
The South American market was valued at USD 11.10 million in 2023 and is expected to reach USD 19.38 million by 2033. This growth is driven by improving healthcare access and the increasing prevalence of respiratory conditions. Additionally, partnerships between local and international manufacturers are expected to enhance market penetration.Middle East & Africa Antiinfective Endotracheal Tube Market Report:
The Middle East and Africa market is projected to grow from USD 62.40 million in 2023 to USD 108.97 million by 2033. This growth is underpinned by expanding healthcare facilities and rising investments in improving medical services. Proactive measures against infection control in emerging markets are likely to contribute to market expansion.Tell us your focus area and get a customized research report.
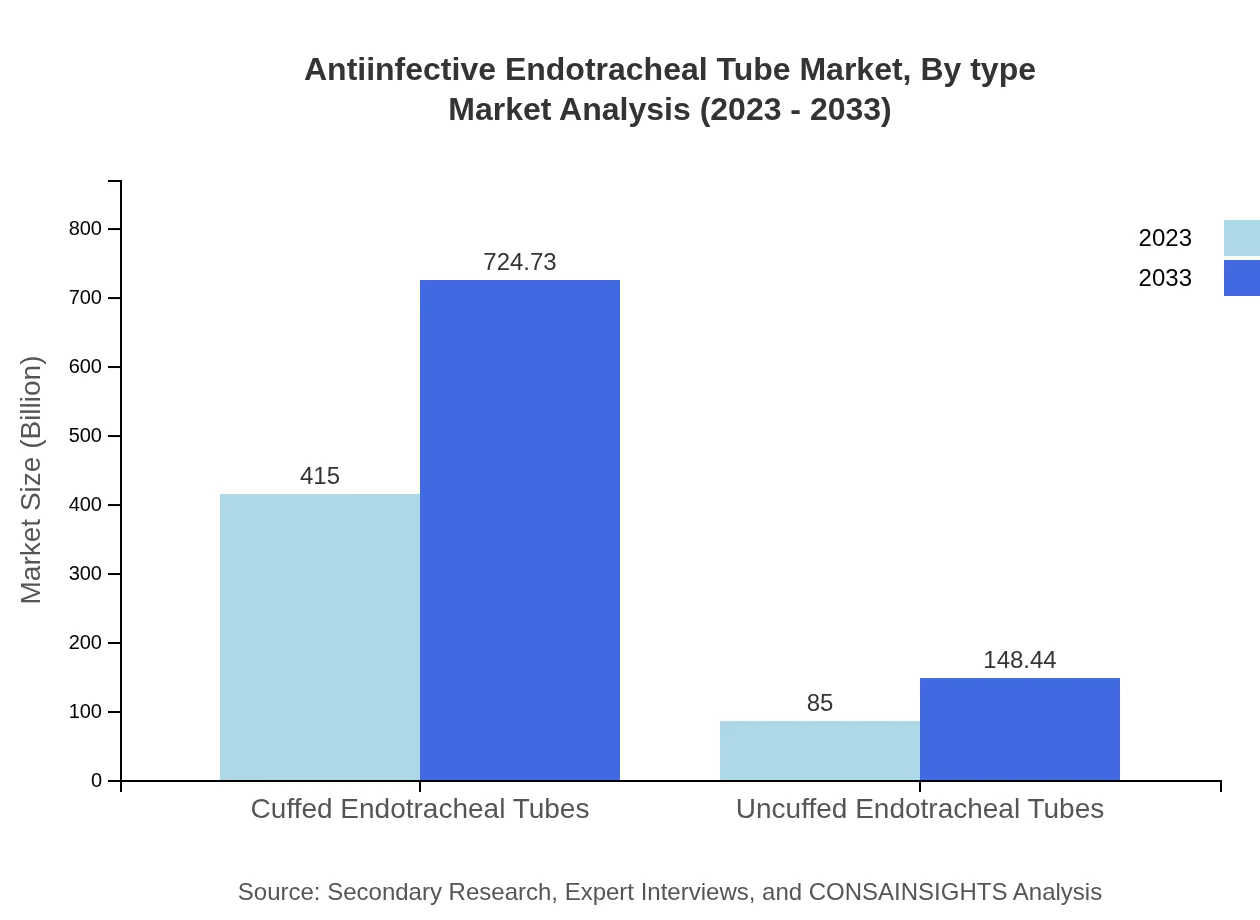
Antiinfective Endotracheal Tube Market Analysis By Type
The market is divided into cuffed and uncuffed endotracheal tubes. The cuffed endotracheal tubes held a significant market share of 83% in 2023, driven by their effectiveness in preventing air leaks during mechanical ventilation. By 2033, this segment is expected to grow from USD 415.00 million to USD 724.73 million. Uncuffed endotracheal tubes, although accounting for a smaller share, are also forecasted to expand their market presence as they are preferred in certain pediatric applications.
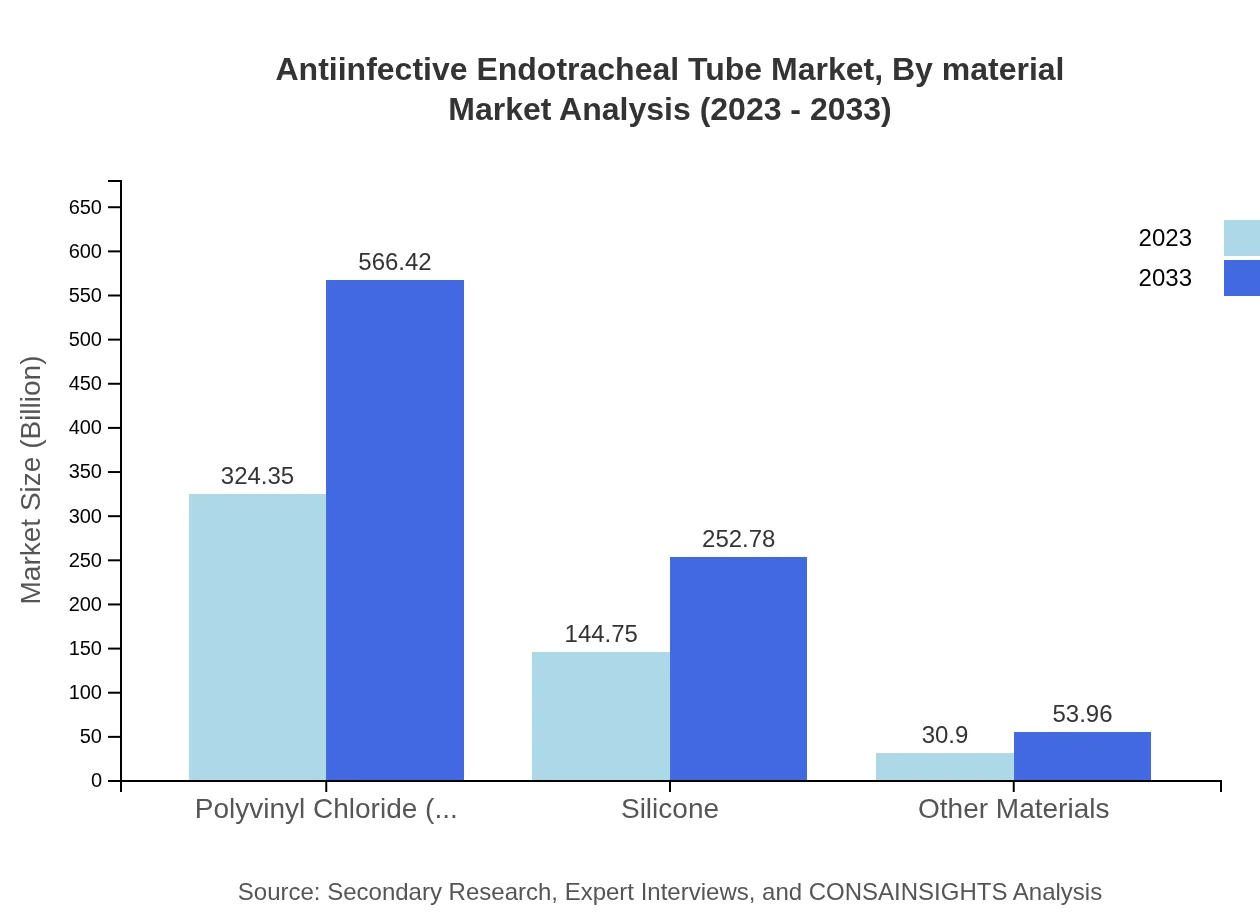
Antiinfective Endotracheal Tube Market Analysis By Material
The anti-infective endotracheal tube market is segmented by material types, primarily focusing on polyvinyl chloride (PVC), silicone, and others. PVC dominates the market, accounting for 64.87% share valued at USD 324.35 million in 2023, expected to reach USD 566.42 million by 2033. Silicone-based tubes are gaining traction due to their biocompatibility, with growth from USD 144.75 million to USD 252.78 million by 2033.
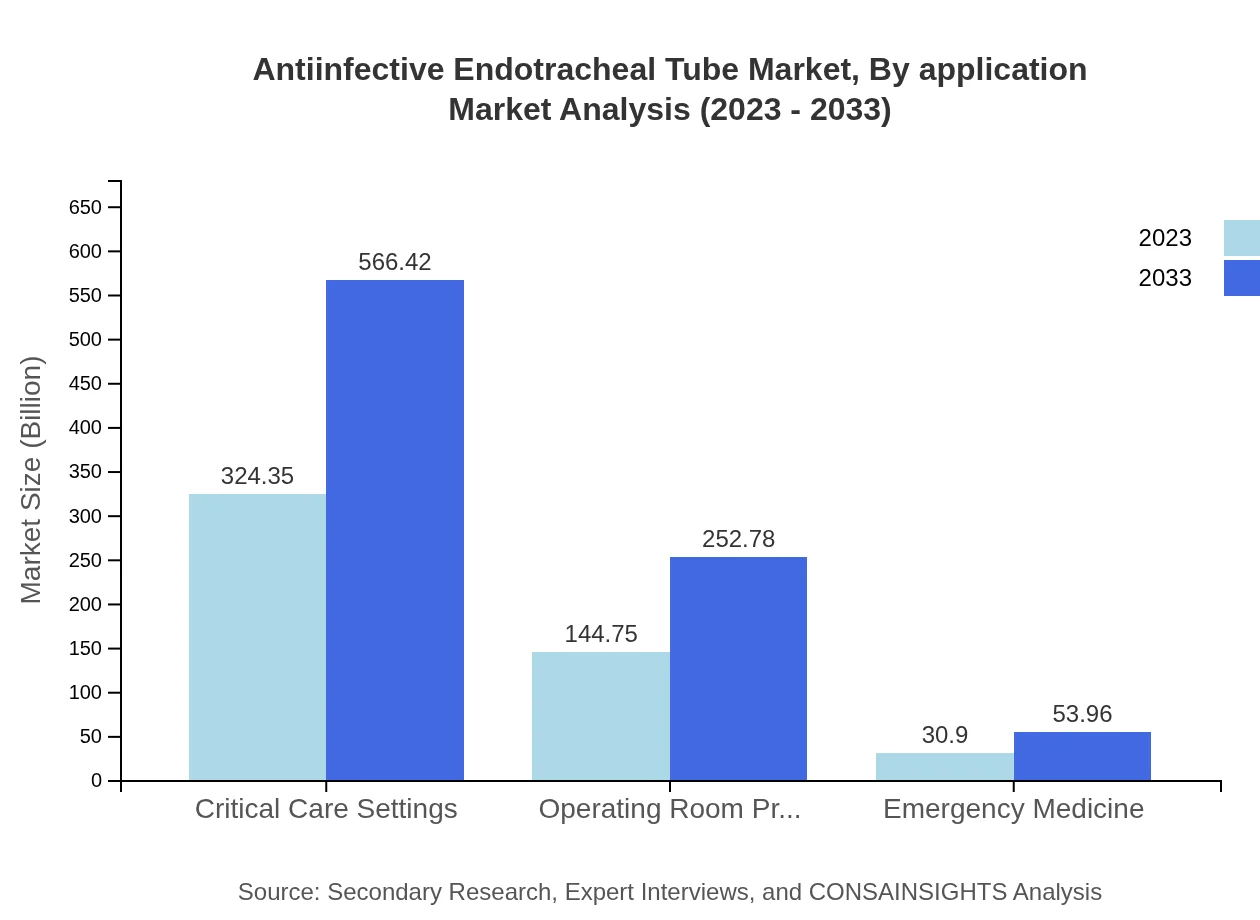
Antiinfective Endotracheal Tube Market Analysis By Application
Applications for anti-infective endotracheal tubes include emergency medicine, operating room procedures, and critical care settings. Critical care settings hold a dominant market share, valued at USD 324.35 million in 2023, and are expected to grow significantly due to an increase in ICU admissions. Operating room procedures are also significant, with expected growth from USD 144.75 million to USD 252.78 million by 2033.
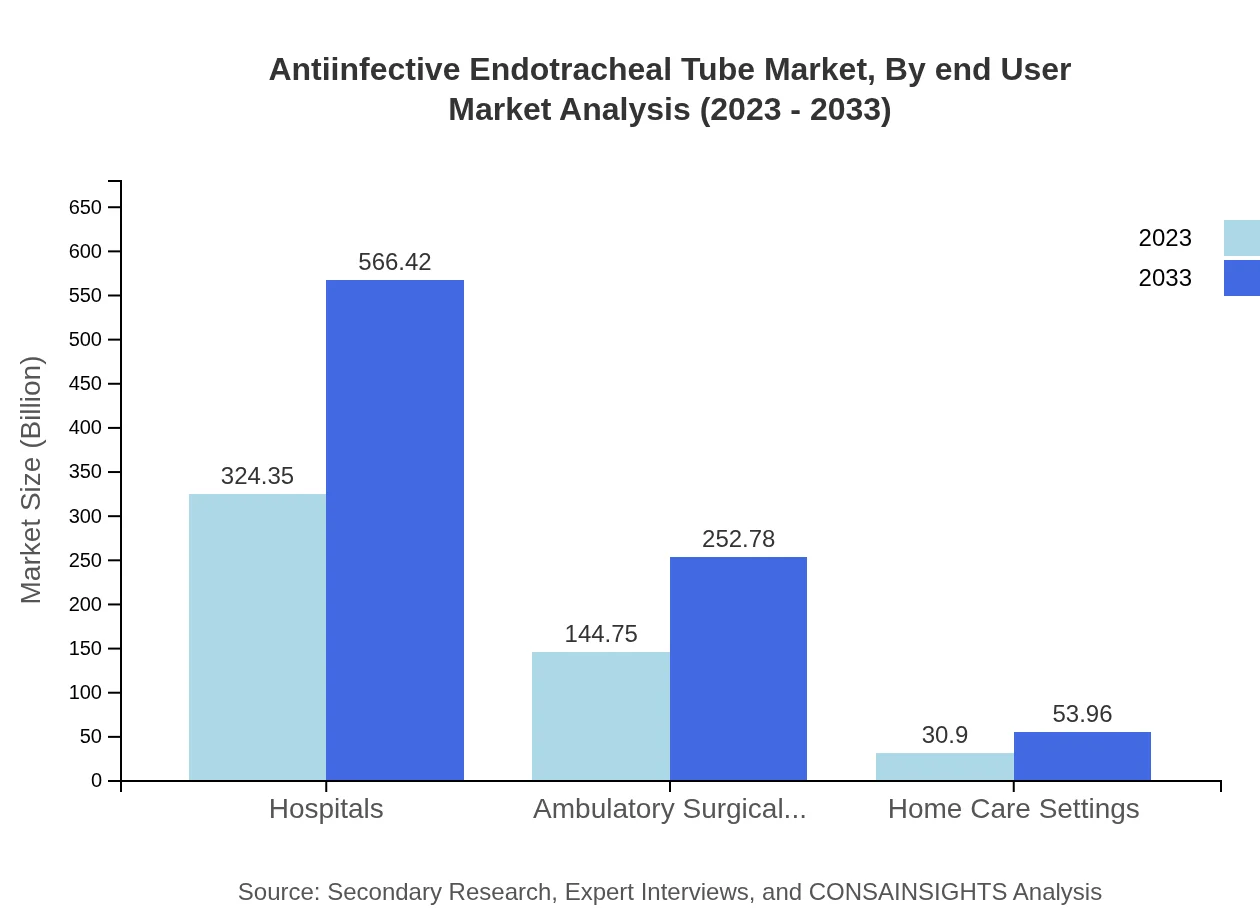
Antiinfective Endotracheal Tube Market Analysis By End User
Key end-users of the anti-infective endotracheal tube market include hospitals, ambulatory surgical centers, and home care settings. Hospitals represent the primary sector, capturing 64.87% of the market with a valuation of USD 324.35 million in 2023 and projected to reach USD 566.42 million by 2033. Ambulatory surgical centers and home care settings also contribute to market growth, although on a smaller scale.
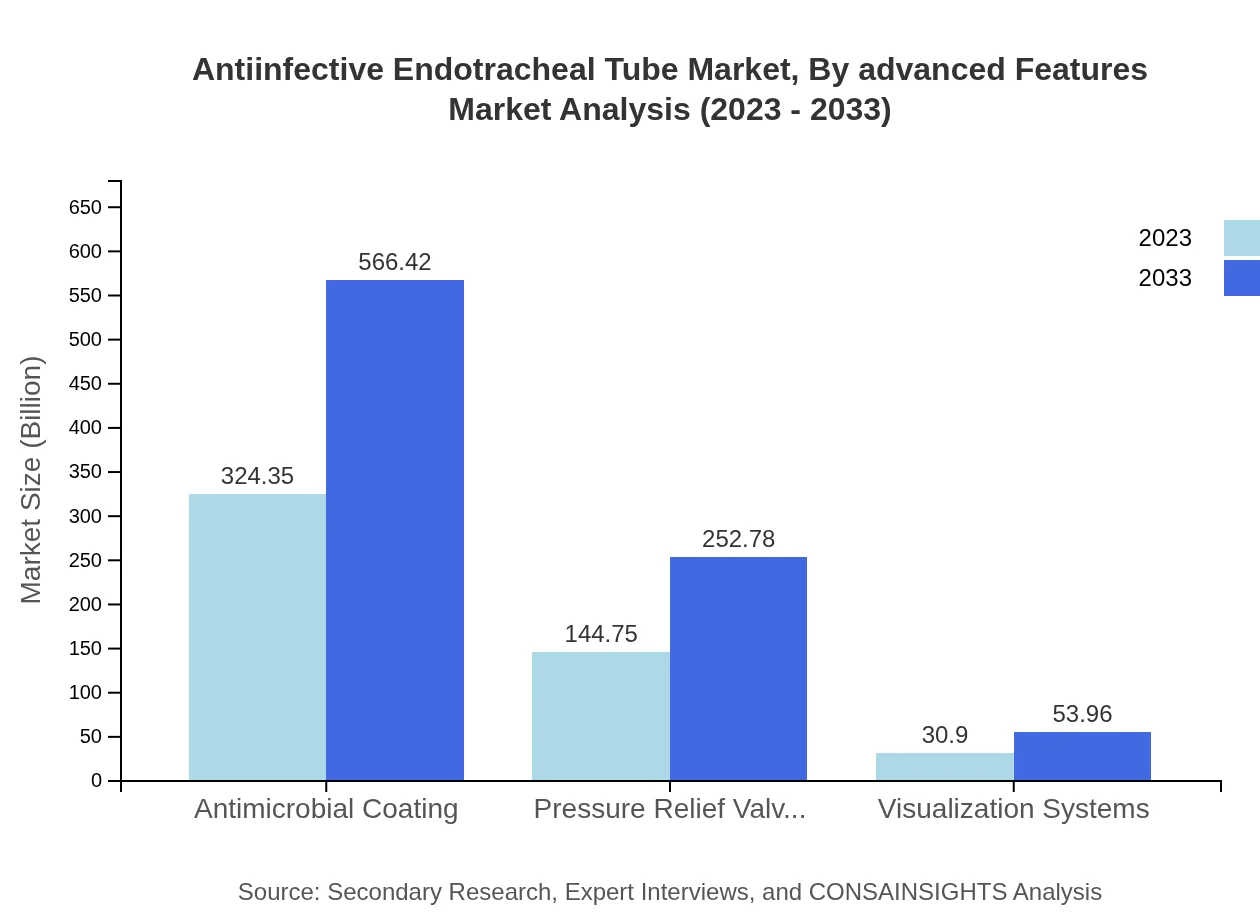
Antiinfective Endotracheal Tube Market Analysis By Advanced Features
Advanced features of anti-infective endotracheal tubes include antimicrobial coatings, pressure relief valves, and visualization systems. Antimicrobial coatings dominate the market, with a size of USD 324.35 million in 2023 and anticipated to reach USD 566.42 million by 2033. Pressure relief valves and visualization systems are also vital, providing enhanced safety during intubation and ventilation procedures.
Anti-infective Endotracheal Tube Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Anti-infective Endotracheal Tube Industry
Medtronic :
A leading global healthcare solutions company, Medtronic specializes in medical devices and therapies, including advanced respiratory solutions like anti-infective endotracheal tubes.Boston Scientific:
Boston Scientific focuses on innovative medical solutions, with a significant presence in respiratory devices and critical care applications.Teleflex:
Teleflex is recognized for its diversified portfolio in medical devices, especially in anesthesiology and critical care, providing essential tools for professionals.Smiths Medical:
Specializing in infusion therapy, respiratory care, and vital care products, Smiths Medical offers a range of anti-infective endotracheal tubes to improve patient safety.Hollister Incorporated:
Hollister is known for its commitment to patient care and extensive solutions in the medical sector, including respiratory management technologies.We're grateful to work with incredible clients.









FAQs
What is the market size of antiinfective Endotracheal Tube?
The antiinfective endotracheal tube market is valued at approximately $500 million in 2023, with a projected CAGR of 5.6% leading to consistent growth over the decade until 2033.
What are the key market players or companies in this antiinfective Endotracheal Tube industry?
Key players in the antiinfective endotracheal tube market include established medical device manufacturers known for their commitment to innovation in respiratory care and infection prevention.
What are the primary factors driving the growth in the antiinfective Endotracheal Tube industry?
The growth in the antiinfective endotracheal tube industry is primarily driven by rising hospitalizations, increasing awareness of infection control, and technological advancements in medical devices.
Which region is the fastest Growing in the antiinfective Endotracheal Tube?
The fastest-growing region for the antiinfective endotracheal tube market is Europe, projected to grow from $181.70 million in 2023 to $317.31 million by 2033.
Does ConsaInsights provide customized market report data for the antiinfective Endotracheal Tube industry?
Yes, ConsaInsights offers customized market report data tailored to the specific needs of clients in the antiinfective endotracheal tube industry for more detailed insights.
What deliverables can I expect from this antiinfective Endotracheal Tube market research project?
Deliverables from this market research will include comprehensive analysis reports, growth forecasts, competitive landscape insights, and detailed regional breakdowns pertinent to the antiinfective endotracheal tube market.
What are the market trends of antiinfective Endotracheal Tube?
Current market trends indicate a shift towards innovative design improvements, increased demand for antimicrobial features, and a growing market share in hospitals and surgical centers.