Antimicrobial Endotracheal Tube Market Report
Published Date: 31 January 2026 | Report Code: antimicrobial-endotracheal-tube
Antimicrobial Endotracheal Tube Market Size, Share, Industry Trends and Forecast to 2033
This report delves into the Antimicrobial Endotracheal Tube market, providing comprehensive insights, forecasts, and analyses from 2023 to 2033. It encompasses market size, segmentation, trends, regional dynamics, and key players within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
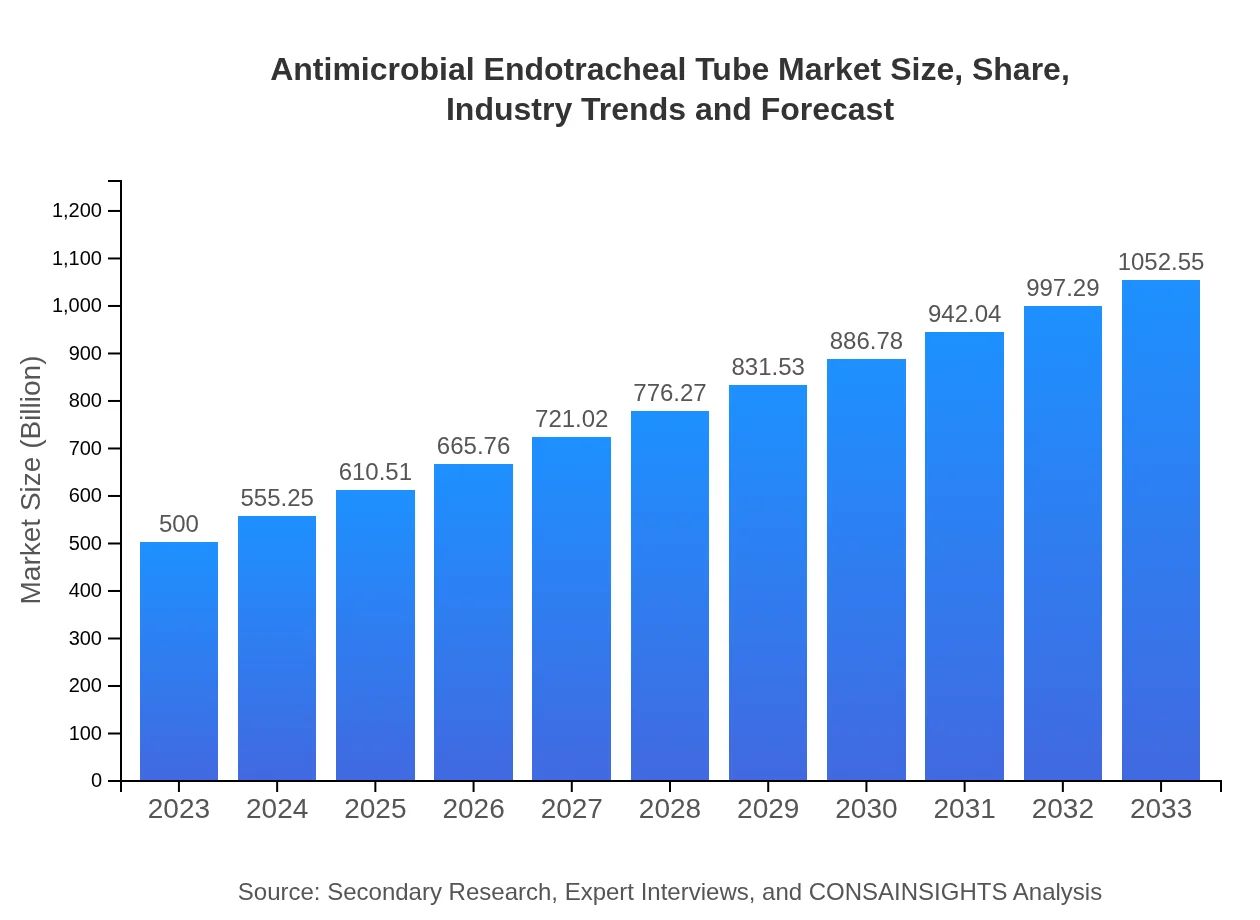
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 7.5% |
| 2033 Market Size | $1052.55 Million |
| Top Companies | Medtronic , Teleflex Incorporated, Smiths Medical, ConvaTec |
| Last Modified Date | 31 January 2026 |
Antimicrobial Endotracheal Tube Market Overview
Customize Antimicrobial Endotracheal Tube Market Report market research report
- ✔ Get in-depth analysis of Antimicrobial Endotracheal Tube market size, growth, and forecasts.
- ✔ Understand Antimicrobial Endotracheal Tube's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Antimicrobial Endotracheal Tube
What is the Market Size & CAGR of Antimicrobial Endotracheal Tube market in 2023-2033?
Antimicrobial Endotracheal Tube Industry Analysis
Antimicrobial Endotracheal Tube Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Antimicrobial Endotracheal Tube Market Analysis Report by Region
Europe Antimicrobial Endotracheal Tube Market Report:
The European market stands at $121.60 million in 2023 and is projected to grow to $255.98 million by 2033. Increasing regulatory standards and healthcare policies aimed at reducing hospital-acquired infections underpin market growth across European nations.Asia Pacific Antimicrobial Endotracheal Tube Market Report:
The Asia Pacific region exhibits a burgeoning market, valued at approximately $99.50 million in 2023, projected to grow to $209.46 million by 2033. Rising healthcare expenditures and an increasing number of surgical procedures drive demand, alongside improvements in healthcare infrastructure.North America Antimicrobial Endotracheal Tube Market Report:
North America registers the largest market share, currently valued at $167.45 million in 2023, anticipated to reach $352.50 million by 2033. This region benefits from advanced healthcare facilities, a high prevalence of chronic respiratory Conditions, and stringent infection prevention protocols.South America Antimicrobial Endotracheal Tube Market Report:
In South America, the market is estimated at $47.25 million in 2023, expected to expand to $99.47 million by 2033. Growing awareness regarding infection control within medical settings will likely augment market growth.Middle East & Africa Antimicrobial Endotracheal Tube Market Report:
The Middle East and Africa market is expected to increase from $64.20 million in 2023 to approximately $135.15 million by 2033. Recent investments in healthcare infrastructure and a heightened focus on infection control measures are significant factors contributing to this growth.Tell us your focus area and get a customized research report.
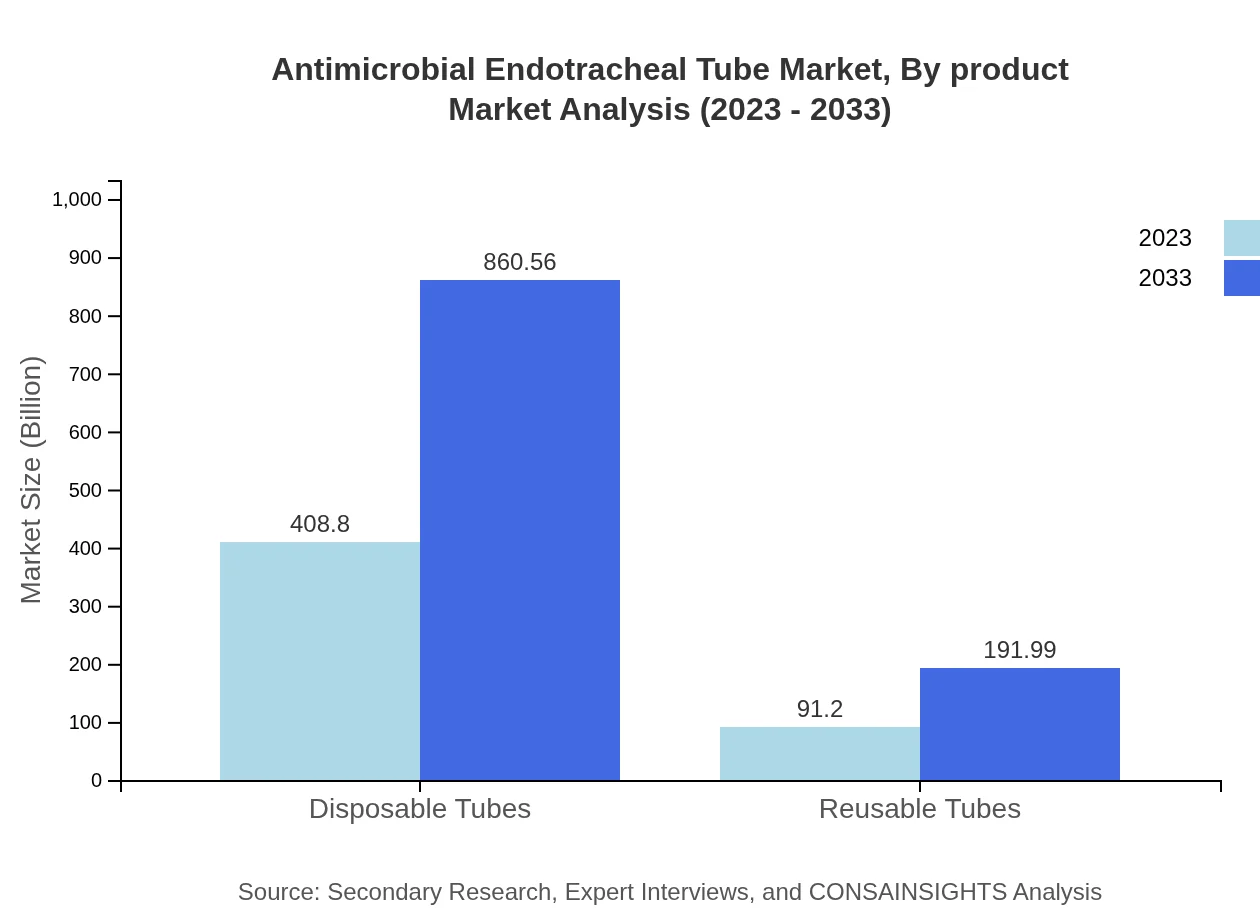
Antimicrobial Endotracheal Tube Market Analysis By Product
Disposable tubes account for a significant portion of the market, valued at $408.80 million in 2023, expected to grow to $860.56 million by 2033. This segment dominates, holding an 81.76% market share in 2023, owing to their hygiene and user-friendly design. Reusable tubes, although smaller, represent an important 18.24% share, with anticipated growth from $91.20 million to $191.99 million in the same period.
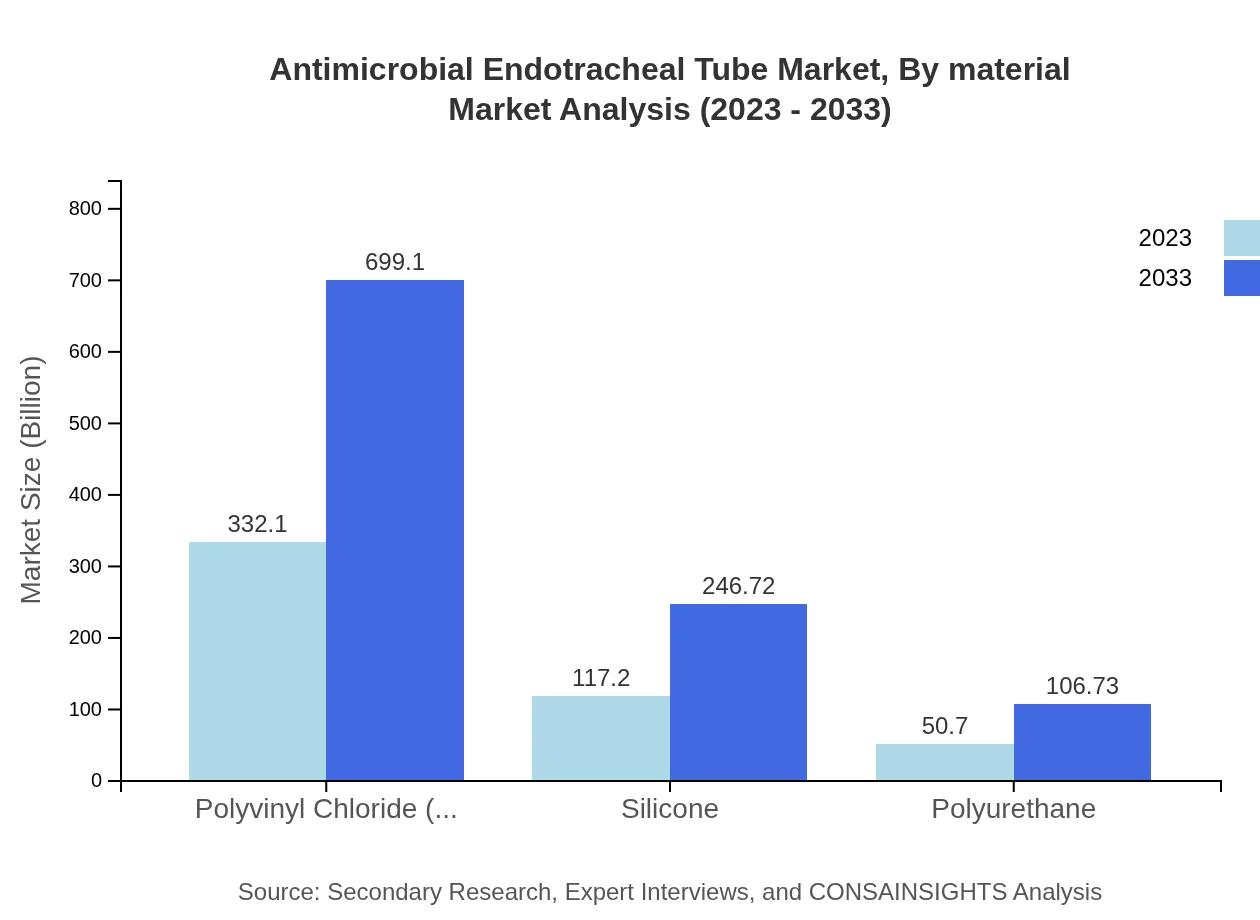
Antimicrobial Endotracheal Tube Market Analysis By Material
Polyvinyl Chloride (PVC) dominates the material segment, expected to grow from $332.10 million in 2023 to $699.10 million in 2033, retaining a 66.42% market share. Silicone tubes, valued at $117.20 million, are projected to reach $246.72 million due to their flexibility and biocompatibility. Polyurethane also holds niche applications with potential growth from $50.70 million to $106.73 million.
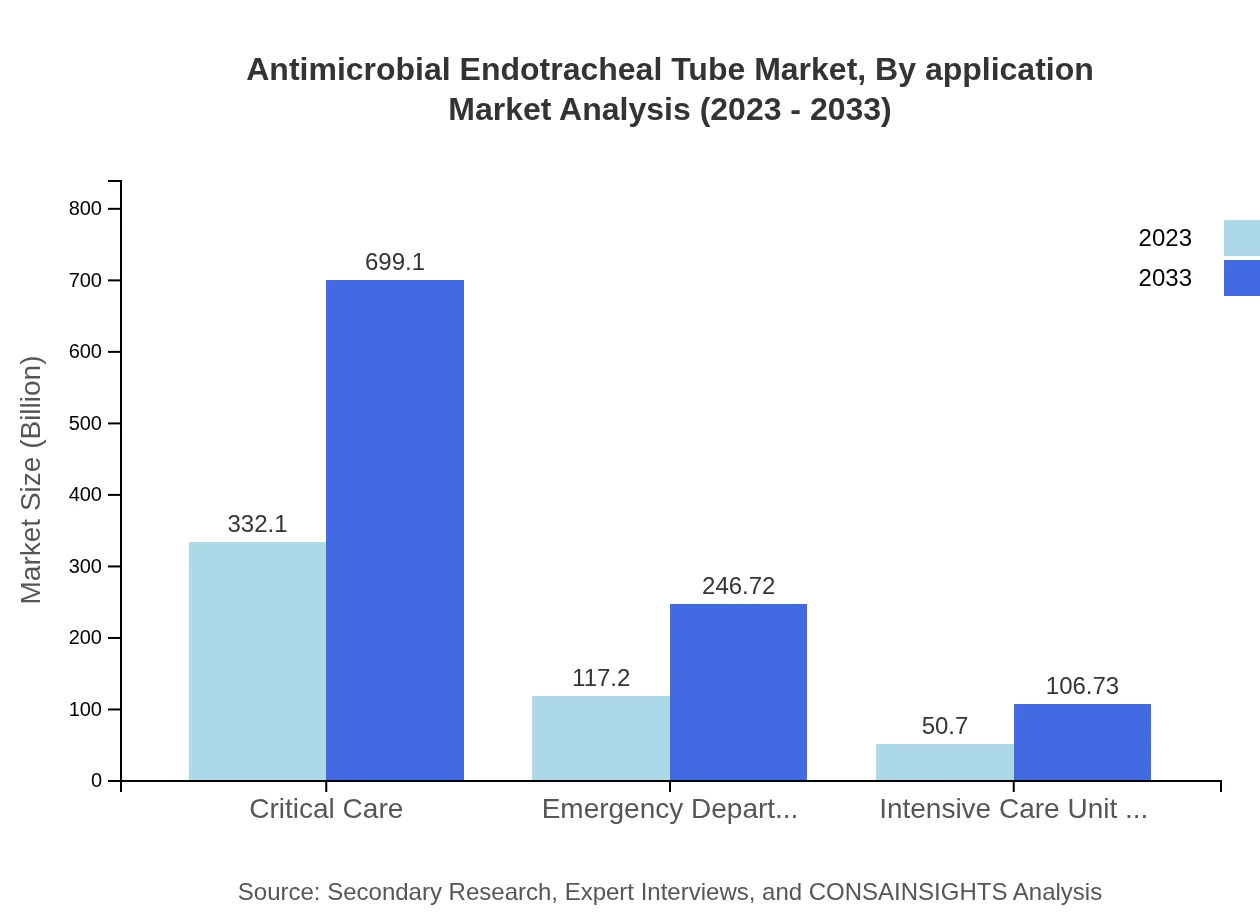
Antimicrobial Endotracheal Tube Market Analysis By Application
The application in critical care represents the largest share, currently at $332.10 million, expected to rise to $699.10 million. The emergency department segment follows, estimated at $117.20 million in 2023 and projected to double by 2033. Intensive care units account for $50.70 million and also exhibit potential for increased market penetration.
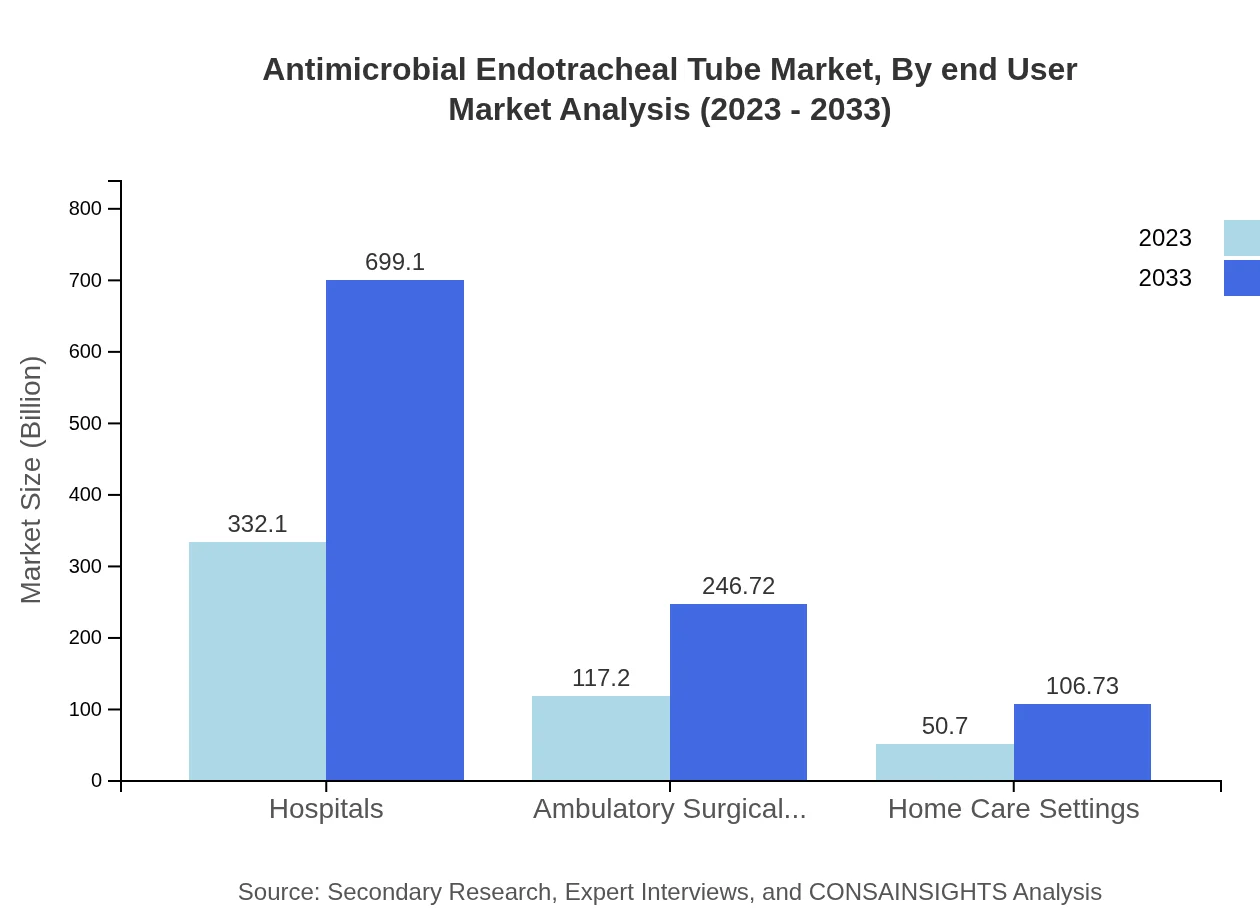
Antimicrobial Endotracheal Tube Market Analysis By End User
Hospitals dominate the end-user segment with $332.10 million in 2023, expected to grow to $699.10 million. Ambulatory surgical centers and home care settings contribute $117.20 million and $50.70 million respectively, reflecting the increasing diversification of healthcare services.
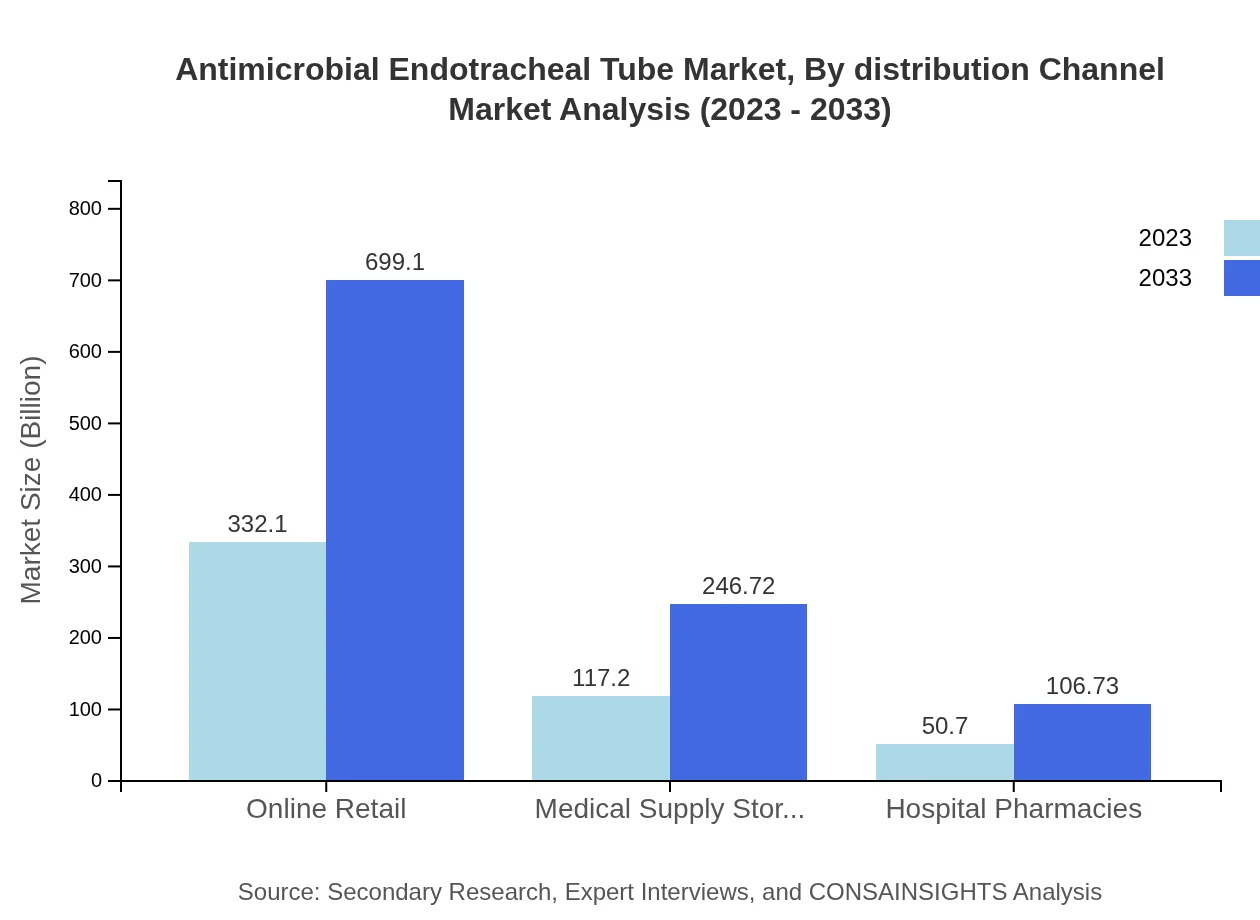
Antimicrobial Endotracheal Tube Market Analysis By Distribution Channel
Online retail and medical supply stores are gaining traction in the distribution channel segment, where online sales are projected to increase from $332.10 million to $699.10 million over the report period. Medical supply stores and hospital pharmacies hold respective shares at smaller dollar values but maintain significant roles in distribution, especially within localized healthcare settings.
Antimicrobial Endotracheal Tube Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Antimicrobial Endotracheal Tube Industry
Medtronic :
A global leader in medical technology, Medtronic offers innovative antimicrobial endotracheal tubes designed to reduce the risk of infections during intubation procedures.Teleflex Incorporated:
Teleflex is renowned for its high-quality medical devices. Their antimicrobial endotracheal tubes are pivotal in improving patient outcomes in critical care.Smiths Medical:
With a strong focus on patient safety, Smiths Medical manufactures a range of antimicrobial endotracheal tubes aimed at enhancing ventilation and minimizing infection risks.ConvaTec:
A key player in pharmaceutical and medical products, ConvaTec develops advanced antimicrobial solutions for ventilatory support.We're grateful to work with incredible clients.









FAQs
What is the market size of antimicrobial endotracheal tubes?
The global antimicrobial endotracheal tube market is estimated to reach $500 million by 2033, growing at a CAGR of 7.5% from 2023. This growth is driven by increasing demand for advanced medical devices and rising healthcare expenditures.
What are the key market players or companies in this antimicrobial endotracheal tube industry?
Key players in the antimicrobial endotracheal tube market include major manufacturers and suppliers that specialize in the production and distribution of medical devices. This includes companies that focus on innovation and quality in respiratory care equipment.
What are the primary factors driving the growth in the antimicrobial endotracheal tube industry?
Growth in the antimicrobial endotracheal tube industry is primarily driven by rising incidences of respiratory diseases, technological advancements in medical devices, and an increase in surgical procedures requiring such equipment, along with a growing emphasis on infection control.
Which region is the fastest Growing in the antimicrobial endotracheal tube market?
The fastest-growing regions in the antimicrobial endotracheal tube market include North America, Europe, and Asia Pacific. In particular, North America is anticipated to witness significant growth, supported by advancements in healthcare technology and high healthcare spending.
Does ConsaInsights provide customized market report data for the antimicrobial endotracheal tube industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the antimicrobial endotracheal tube industry, enabling clients to gain insights into niche market areas and make informed decisions.
What deliverables can I expect from this antimicrobial endotracheal tube market research project?
Deliverables from the antimicrobial endotracheal tube market research project typically include detailed market analysis, forecasts, regional data, competitive landscape insights, and strategic recommendations based on current market trends.
What are the market trends of antimicrobial endotracheal tubes?
Market trends indicate a growing focus on antimicrobial technologies, increased adoption of disposable tubes, and a shift toward home care settings. Additionally, a rise in online retailing is expanding market access to various customer segments.