Antinuclear Antibody Test Market Report
Published Date: 31 January 2026 | Report Code: antinuclear-antibody-test
Antinuclear Antibody Test Market Size, Share, Industry Trends and Forecast to 2033
This market report provides an in-depth analysis of the Antinuclear Antibody Test market, covering key insights, market dynamics, forecasts from 2023 to 2033, and detailed segmentations, including regional analysis and technological advancements.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
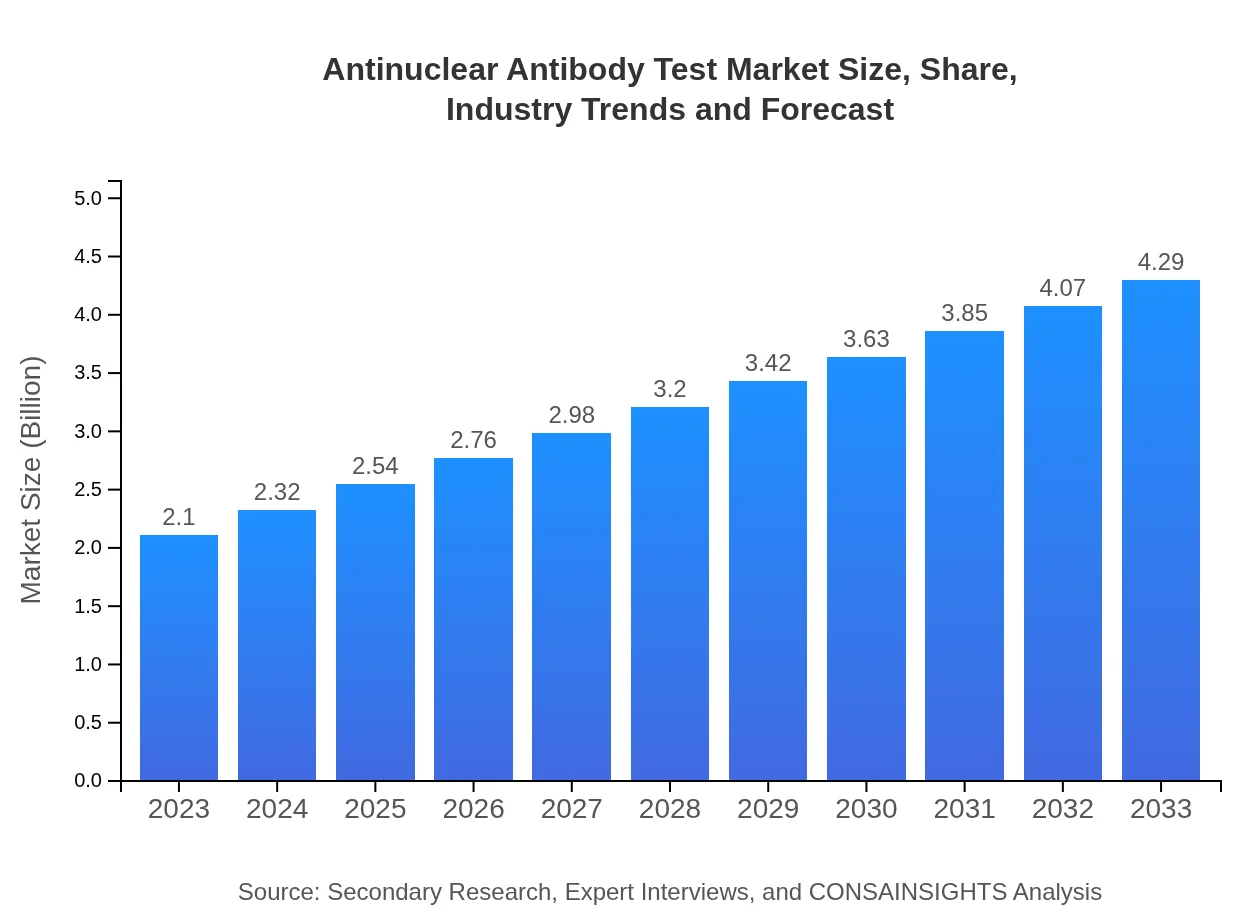
| 2023 Market Size | $2.10 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $4.29 Billion |
| Top Companies | Abbott Laboratories, Thermo Fisher Scientific Inc., Roche Diagnostics, Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Antinuclear Antibody Test Market Overview
Customize Antinuclear Antibody Test Market Report market research report
- ✔ Get in-depth analysis of Antinuclear Antibody Test market size, growth, and forecasts.
- ✔ Understand Antinuclear Antibody Test's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Antinuclear Antibody Test
What is the Market Size & CAGR of Antinuclear Antibody Test market in 2023 and 2033?
Antinuclear Antibody Test Industry Analysis
Antinuclear Antibody Test Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Antinuclear Antibody Test Market Analysis Report by Region
Europe Antinuclear Antibody Test Market Report:
The European Antinuclear Antibody Test market is projected to increase from $0.77 billion in 2023 to $1.56 billion in 2033. This growth is fueled by rising healthcare investments, technological advancements, and collaborative research efforts across the region.Asia Pacific Antinuclear Antibody Test Market Report:
The Asia Pacific region is expected to witness considerable growth in the Antinuclear Antibody Test market, with a projected market size of $0.72 billion by 2033, up from $0.35 billion in 2023. Factors driving this growth include rising healthcare expenditure, increasing demand for advanced diagnostic tests, and the prevalence of autoimmune diseases.North America Antinuclear Antibody Test Market Report:
North America dominates the Antinuclear Antibody Test market, with an estimated market size of $1.41 billion by 2033, compared to $0.69 billion in 2023. The region benefits from a robust healthcare infrastructure, higher incidence rates of autoimmune diseases, and advanced research capabilities.South America Antinuclear Antibody Test Market Report:
In South America, the Antinuclear Antibody Test market is anticipated to grow from $0.18 billion in 2023 to $0.38 billion in 2033. The growth is supported by increasing awareness regarding autoimmune diseases and access to improved diagnostic technologies.Middle East & Africa Antinuclear Antibody Test Market Report:
The Middle East and Africa region is expected to grow modestly, with a market size of $0.22 billion by 2033, up from $0.11 billion in 2023. Growth here is driven by improving healthcare systems and increasing awareness of testing for autoimmune diseases.Tell us your focus area and get a customized research report.
Antinuclear Antibody Test Market Analysis By Product Type
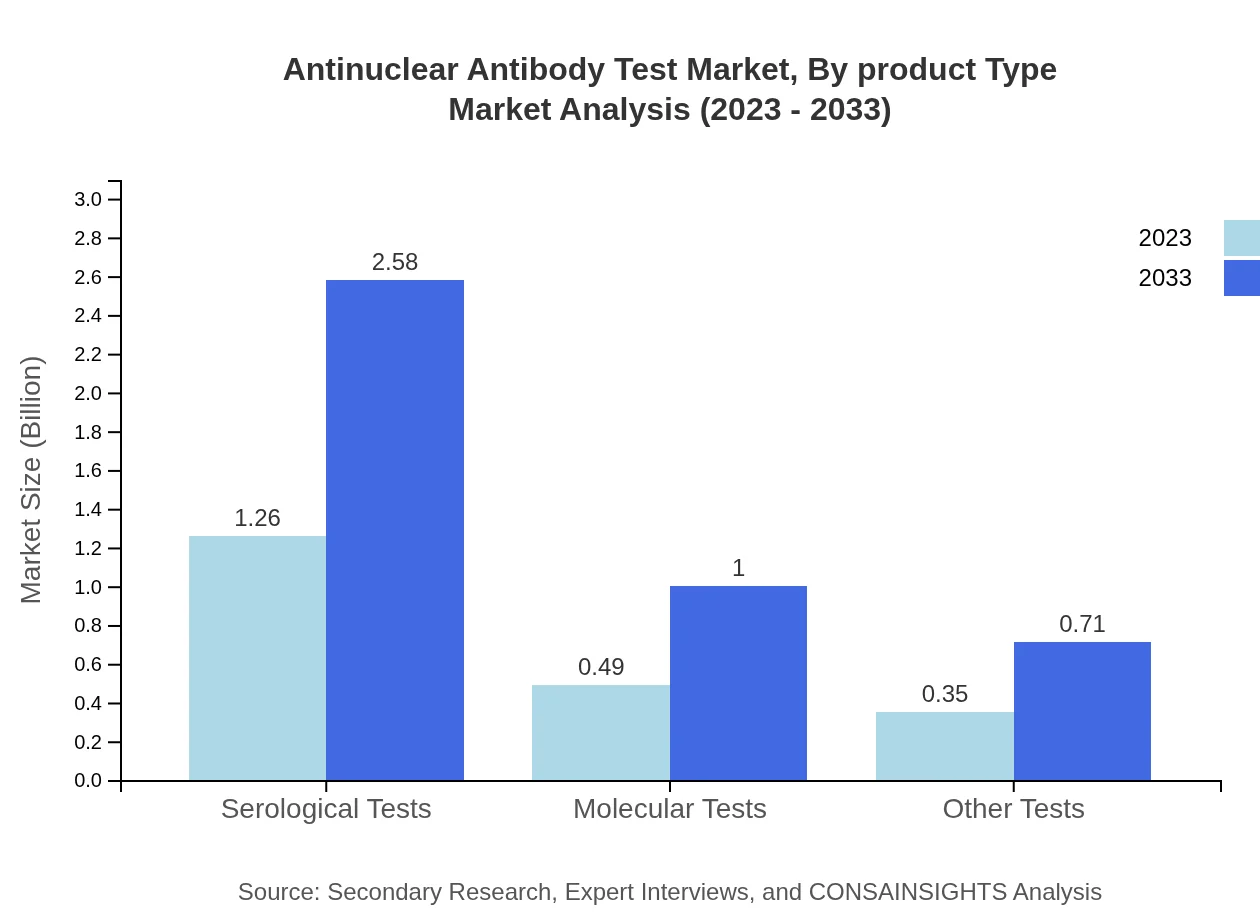
The Antinuclear Antibody Test market by product type includes serological tests, molecular tests, and others like manual and automated testing. Serological tests hold the largest share at 60.14% in 2023, with projected growth reflecting their critical role in diagnosing autoimmune diseases. Molecular tests, while smaller at 23.3%, are gaining traction due to their accuracy and rapid results.
Antinuclear Antibody Test Market Analysis By Testing Method
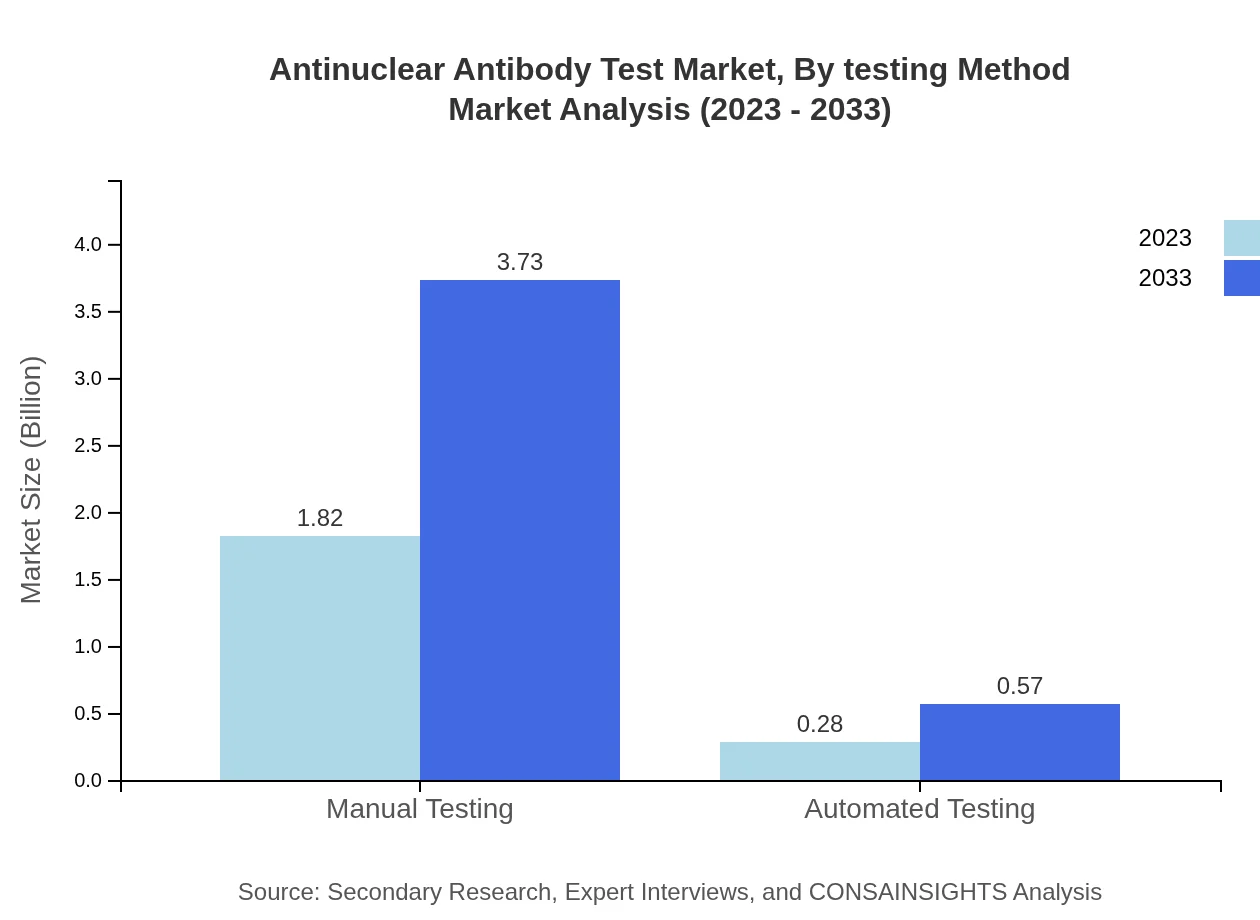
The market is segmented into manual and automated testing methodologies, with manual testing currently dominating at 86.78% market share. However, automated testing is expected to grow significantly, reflecting a demand for speed and efficiency in laboratory settings.
Antinuclear Antibody Test Market Analysis By End Users
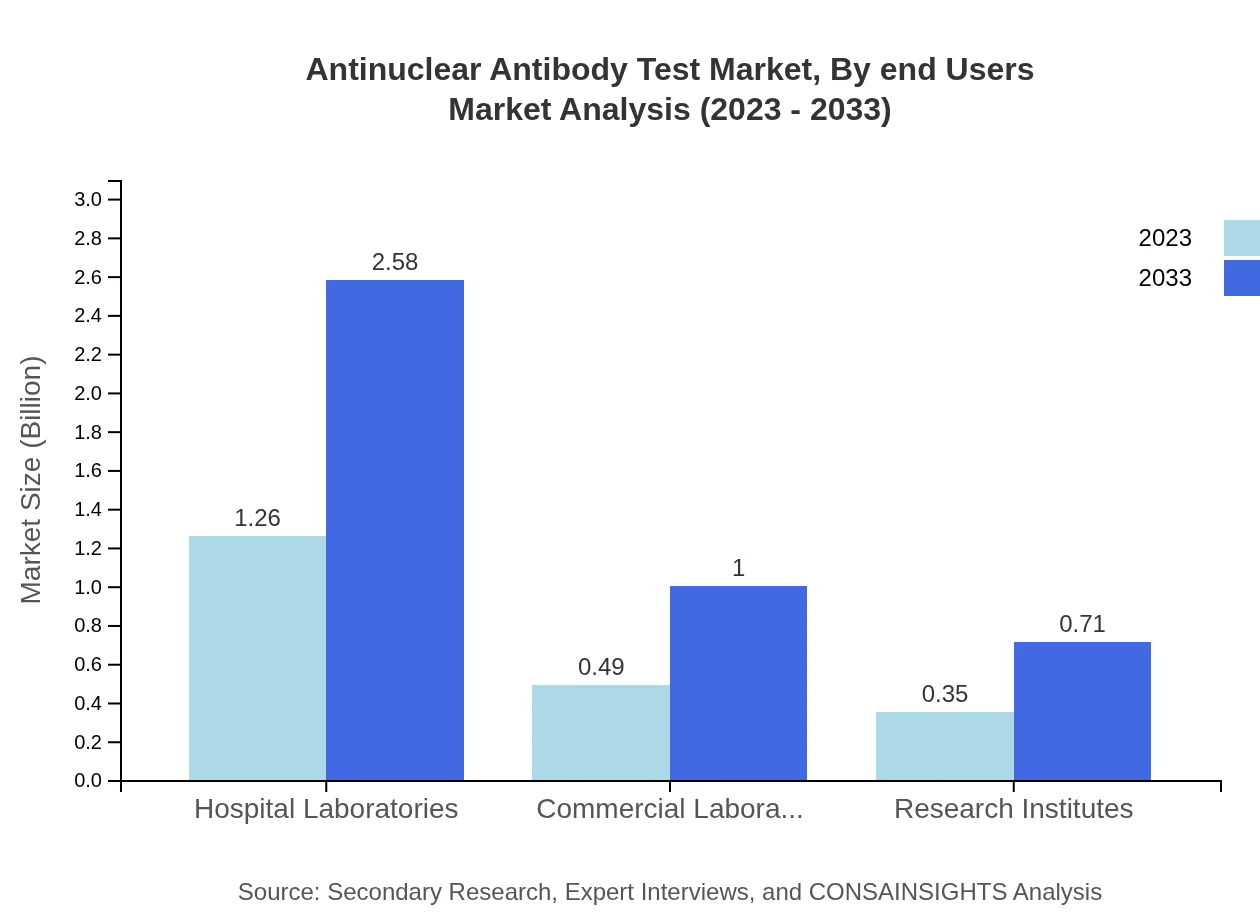
End users include hospital laboratories, commercial laboratories, and research institutes. Hospital laboratories represent the largest segment, with a market size of $1.26 billion in 2023. Commercial laboratories are also influential, with a market growing to $1.00 billion by 2033, indicating a progressive trend toward outsourcing diagnostic testing.
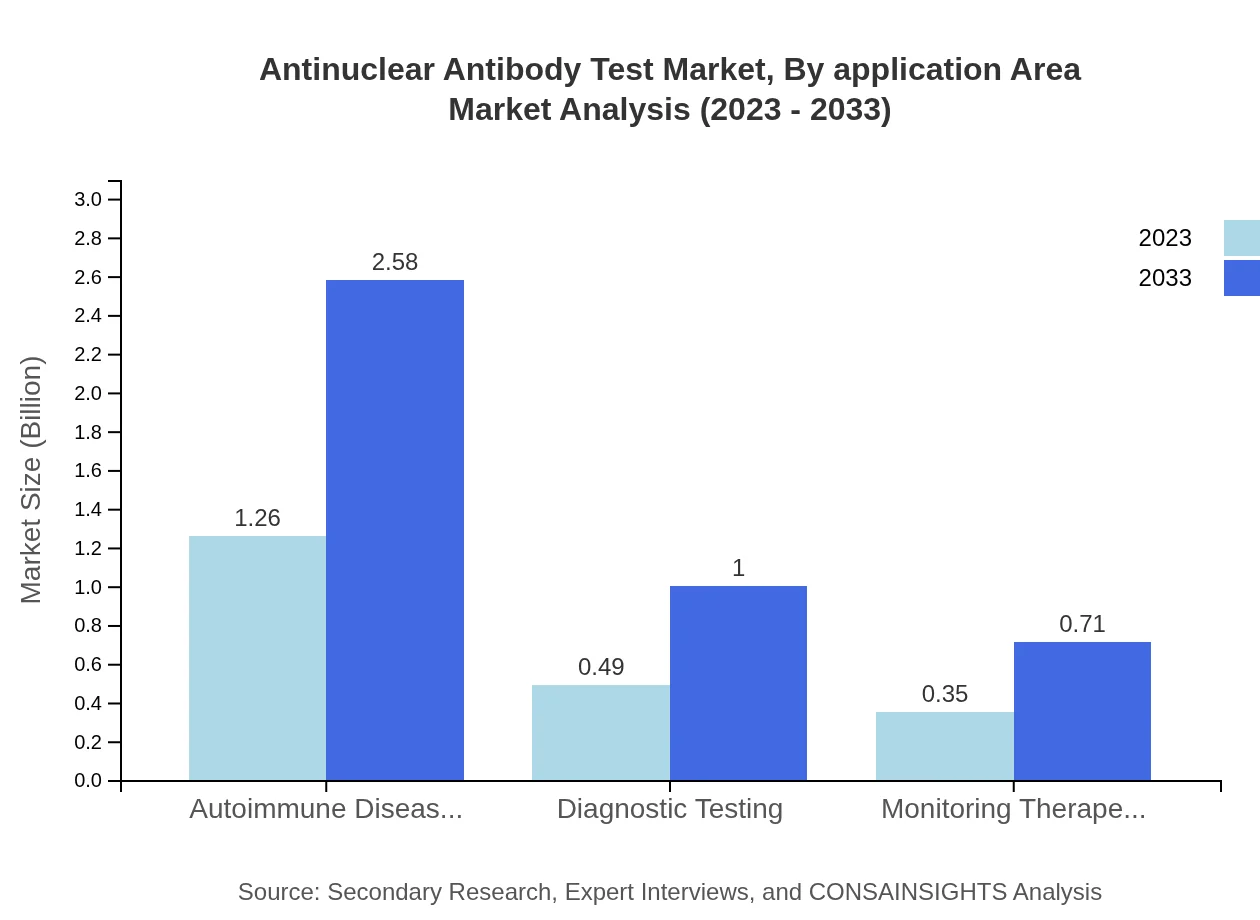
Antinuclear Antibody Test Market Analysis By Application Area
The application area includes autoimmune diseases, diagnostic testing, and monitoring therapeutic response. Autoimmune diseases segment accounts for 60.14% of the market and is the primary focus of the ANA tests, underlining the necessity of rigorous diagnostic protocols in managing these conditions.
Antinuclear Antibody Test Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Antinuclear Antibody Test Industry
Abbott Laboratories:
A major player in the global diagnostics industry, known for innovative testing solutions for various diseases, including autoimmune conditions.Thermo Fisher Scientific Inc.:
A leader in laboratory services and diagnostic kits, offering a comprehensive range of tests and reagents for ANA testing.Roche Diagnostics:
This company is renowned for its advanced diagnostic products and solutions that enhance the accuracy and efficiency of autoimmune disease testing.Siemens Healthineers:
Provides state-of-the-art diagnostic solutions and technologies in laboratory diagnostics, contributing significantly to ANA test advancements.We're grateful to work with incredible clients.









FAQs
What is the market size of antinuclear Antibody Test?
The antinuclear antibody test market is currently valued at approximately $2.1 billion in 2023, and it is projected to grow at a CAGR of 7.2% over the next decade, indicating robust expansion and increasing demand.
What are the key market players or companies in this antinuclear Antibody Test industry?
Key players in the antinuclear antibody test market include leading diagnostic companies and clinical laboratories. They are continuously innovating and enhancing their test offerings to maintain competitive advantage.
What are the primary factors driving the growth in the antinuclear Antibody Test industry?
The growth is driven by the rising prevalence of autoimmune diseases, increasing awareness among healthcare professionals, advancements in diagnostic technologies, and the growing focus on early disease detection.
Which region is the fastest Growing in the antinuclear Antibody Test?
The fastest-growing region is Europe, projected to increase from $0.77 billion in 2023 to $1.56 billion by 2033, reflecting a strong demand for diagnostic testing and management of autoimmune diseases.
Does ConsaInsights provide customized market report data for the antinuclear Antibody Test industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs, including market trends, forecasts, and competitive analysis in the antinuclear antibody test sector.
What deliverables can I expect from this antinuclear Antibody Test market research project?
Deliverables include comprehensive market analysis reports, competitive landscape assessments, segmentation data, and regional insights to inform strategic decision-making.
What are the market trends of antinuclear Antibody Test?
Market trends include a shift towards automated testing, increased adoption of serological tests, and enhanced focus on identifying specific autoimmune disorders to improve diagnostic accuracy.