Antivenoms Market Report
Published Date: 31 January 2026 | Report Code: antivenoms
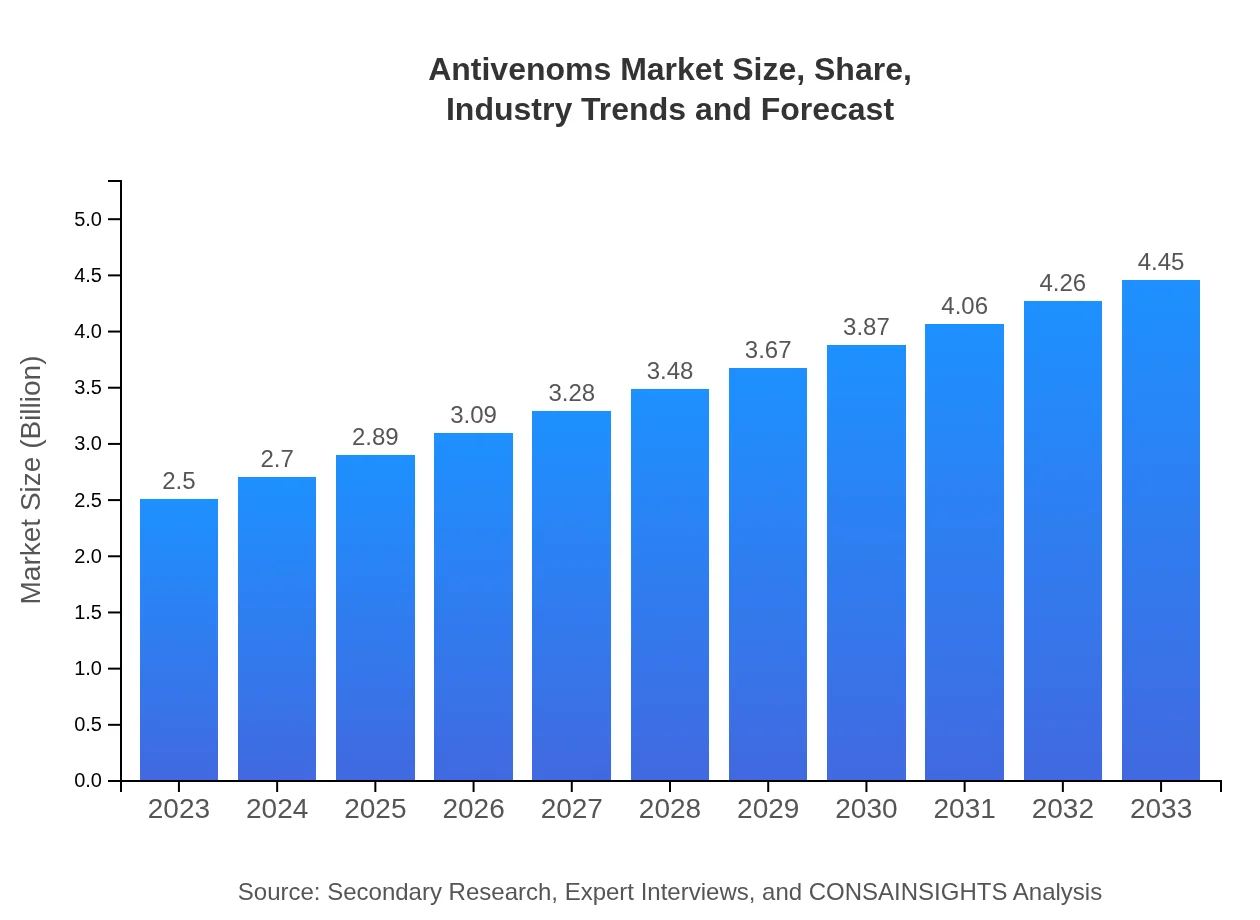
Antivenoms Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Antivenoms market, covering insights into market size, growth trends, regional analysis, and industry dynamics from 2023 to 2033. It also examines key segments and forecasts future developments in this vital healthcare sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $4.45 Billion |
| Top Companies | Baxter International, Serum Institute of India, CSL Limited, Haffkine Bio-Pharmaceutical Corporation Limited |
| Last Modified Date | 31 January 2026 |
Antivenoms Market Overview
Customize Antivenoms Market Report market research report
- ✔ Get in-depth analysis of Antivenoms market size, growth, and forecasts.
- ✔ Understand Antivenoms's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Antivenoms
What is the Market Size & CAGR of Antivenoms market in 2023?
Antivenoms Industry Analysis
Antivenoms Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Antivenoms Market Analysis Report by Region
Europe Antivenoms Market Report:
Europe's antivenoms market is expected to increase from $0.67 billion in 2023 to $1.19 billion by 2033, owing to stringent regulations ensuring product safety and governmental health policies focusing on public health awareness. Increased research activities around immunotherapy further aid market growth.Asia Pacific Antivenoms Market Report:
The Antivenoms market in the Asia Pacific is expected to grow from $0.50 billion in 2023 to $0.90 billion by 2033, driven by a high incidence of snake bites and government initiatives to improve healthcare access. Increasing awareness and educational campaigns are enhancing patient access to critical antivenom treatments.North America Antivenoms Market Report:
The North American market is anticipated to grow significantly, from $0.83 billion in 2023 to $1.48 billion by 2033. The rise in associated healthcare spending and advancements in clinical treatment protocols are key drivers. Enhanced diagnostic facilities also facilitate faster and effective treatment.South America Antivenoms Market Report:
In South America, the market is projected to increase from $0.16 billion in 2023 to $0.28 billion by 2033, primarily due to a rise in venomous snake species and a push from local health organizations to enhance treatment availability in rural areas. Focused initiatives improve the overall health care response.Middle East & Africa Antivenoms Market Report:
The market in the Middle East and Africa will see growth from $0.34 billion in 2023 to $0.61 billion by 2033, buoyed by rising health awareness and infrastructural improvements in healthcare systems, which are critical for treatment accessibility.Tell us your focus area and get a customized research report.
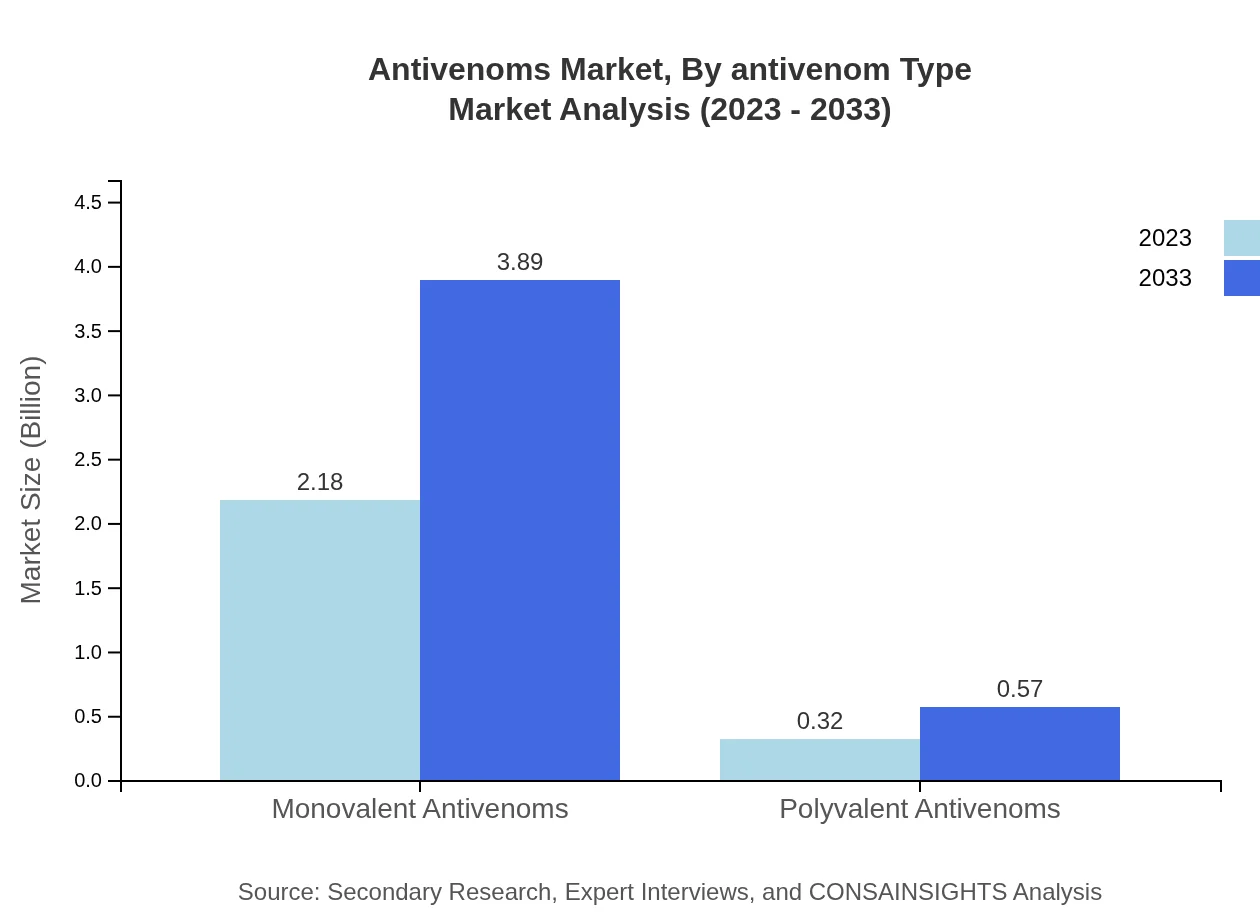
Antivenoms Market Analysis By Antivenom Type
The antivenoms market is primarily segmented into monovalent and polyvalent antivenoms. Monovalent antivenoms dominate the market, holding 87.27% share as of 2023, growing from $2.18 billion in 2023 to $3.89 billion by 2033. Polyvalent antivenoms, although smaller in market share, are also significant, expected to rise from $0.32 billion in 2023 to $0.57 billion by 2033, constituting 12.73% during the same period.
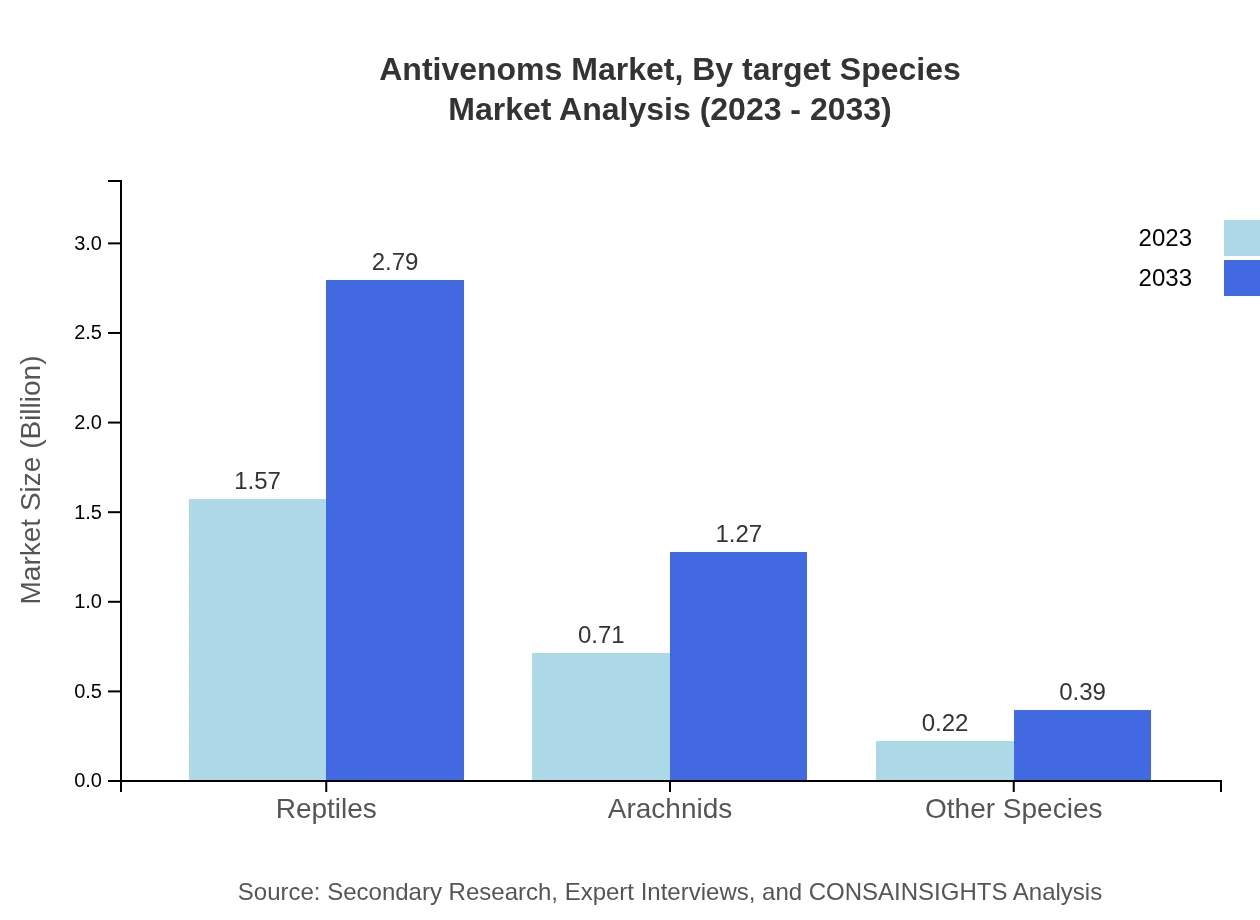
Antivenoms Market Analysis By Target Species
In the antivenom market segmented by target species, products aimed at treating reptile bites account for 62.7% of the market share, projected to grow from $1.57 billion in 2023 to $2.79 billion by 2033. Treatments for arachnid bites are set to increase from $0.71 billion to $1.27 billion, holding a share of 28.43%, while other species contribute with a share of 8.87%.
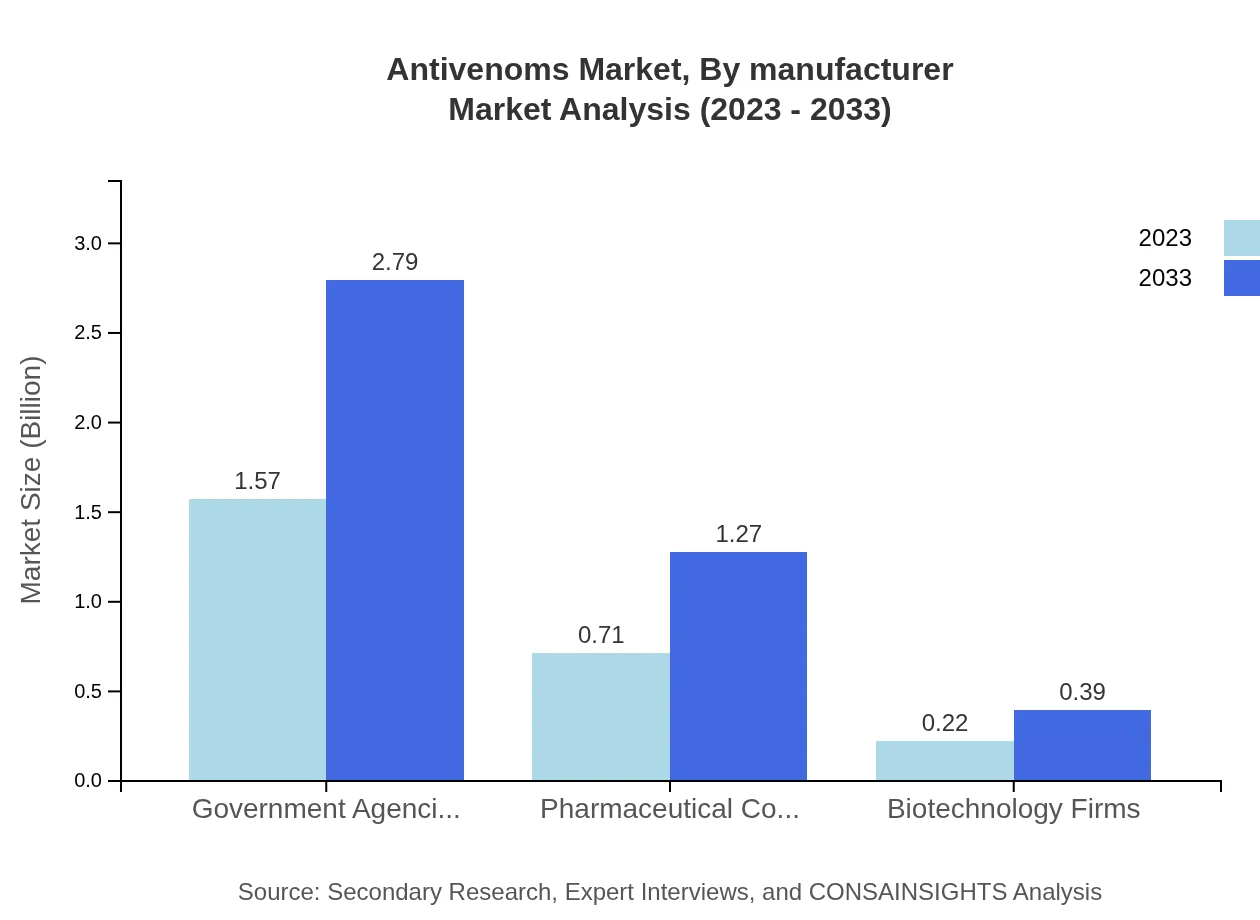
Antivenoms Market Analysis By Manufacturer
The antivenoms market is influenced significantly by various stakeholders, including government agencies (62.7% market share with $1.57 billion expected to grow to $2.79 billion) and pharmaceutical companies (28.43% share, growing from $0.71 billion to $1.27 billion). Biotechnology firms, while smaller, also play a role with an 8.87% share, projected to reach $0.39 billion.
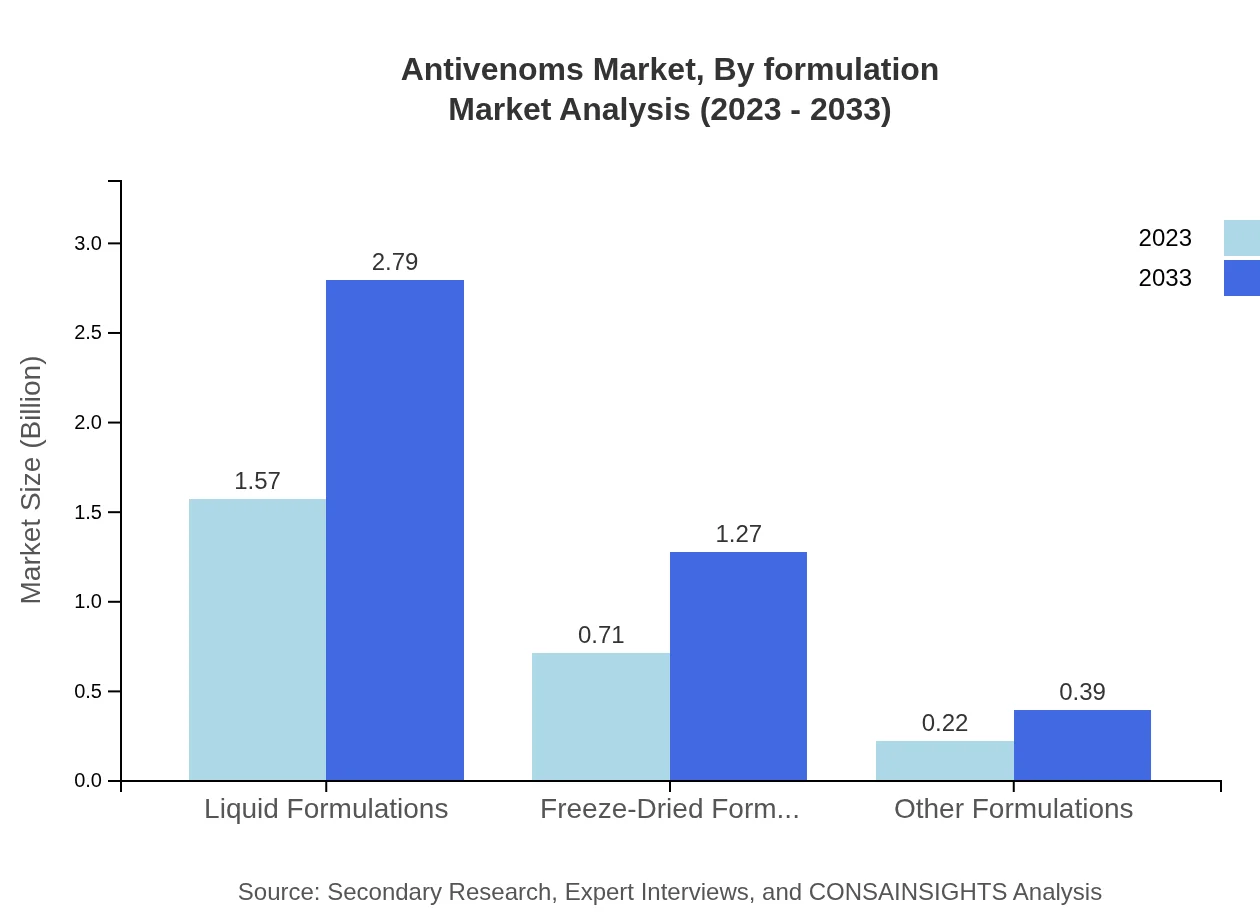
Antivenoms Market Analysis By Formulation
The formulations used in antivenoms primarily include liquid formulations (62.7% share, from $1.57 billion to $2.79 billion) and freeze-dried formulations (28.43% share, from $0.71 billion to $1.27 billion). Other formulations contribute 8.87% with growth from $0.22 billion to $0.39 billion.
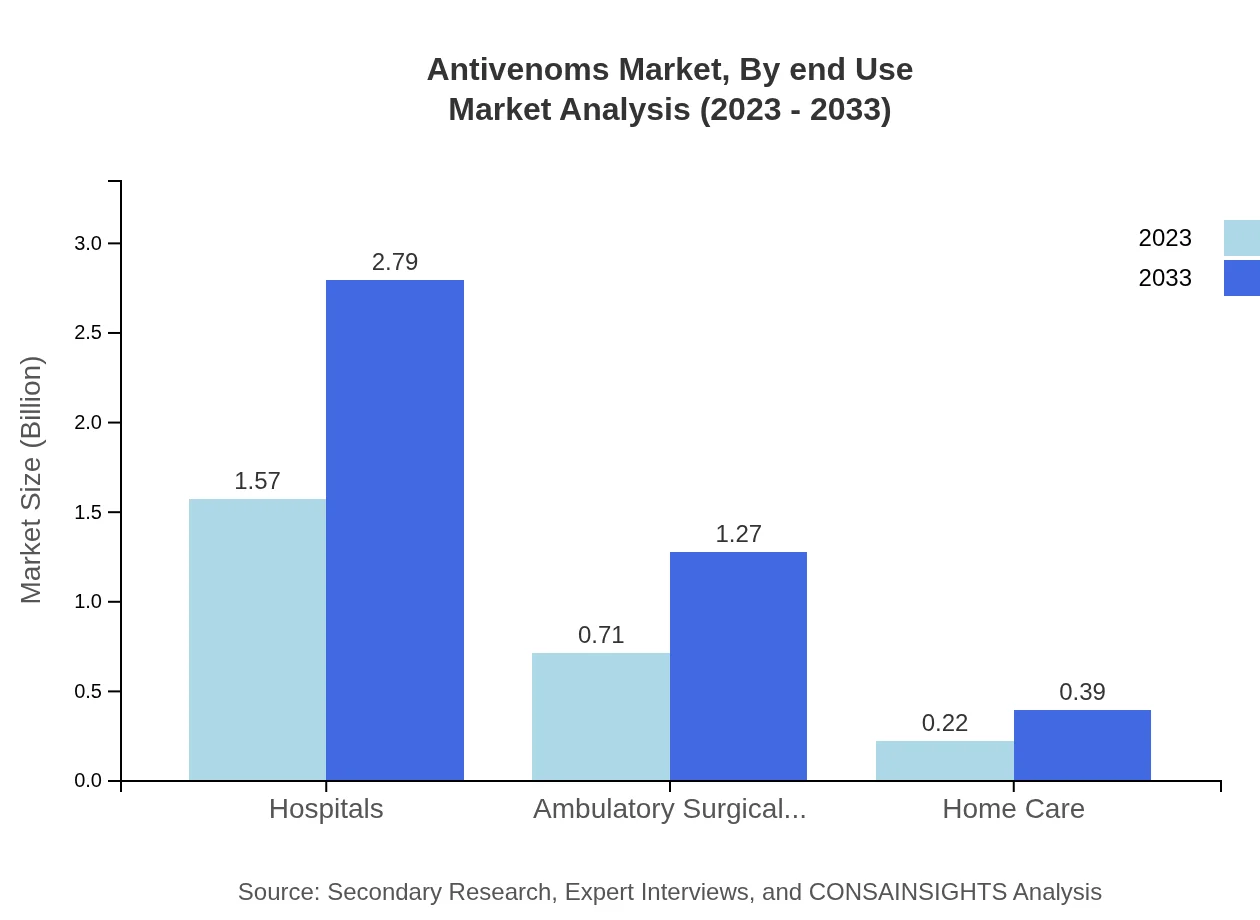
Antivenoms Market Analysis By End Use
By end-use, hospitals remain the primary consumers of antivenoms, holding a 62.7% market share projected to grow from $1.57 billion to $2.79 billion by 2033. Ambulatory surgical centers and home care segments contribute to 28.43% and 8.87% share, respectively, highlighting the expanded accessibility of antivenom treatments.
Antivenoms Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Antivenoms Industry
Baxter International:
A leading pharmaceutical company with a strong focus on developing and producing antivenoms, including monovalent and polyvalent products responsible for treating envenomations.Serum Institute of India:
One of the largest manufacturers globally, focusing on providing affordable antivenoms for various regions, enhancing accessibility to life-saving treatments in low-income areas.CSL Limited:
An innovative biotechnology company, CSL Limited is renowned for its research in antivenom development, aiming to improve effectiveness and safety across various product lines.Haffkine Bio-Pharmaceutical Corporation Limited:
A state-owned company in India, focused on producing high-quality antivenoms for local needs, addressing unique regional challenges in envenomation care.We're grateful to work with incredible clients.









FAQs
What is the market size of antivenoms?
The antivenoms market is valued at approximately $2.5 billion in 2023, with a projected compound annual growth rate (CAGR) of 5.8% over the next decade, highlighting significant growth potential.
What are the key market players or companies in the antivenoms industry?
Key players in the antivenoms market include major pharmaceutical companies and biotechnology firms specializing in venom research and antivenom production, focusing on safety, efficacy, and accessibility of treatments.
What are the primary factors driving the growth in the antivenoms industry?
Growth in the antivenoms market is primarily driven by the rising incidence of snakebites, increased awareness of venomous species, advancements in medical technology, and supportive government initiatives in healthcare.
Which region is the fastest Growing in the antivenoms market?
North America is the fastest-growing region in the antivenoms market, projected to expand from $0.83 billion in 2023 to $1.48 billion by 2033, fueled by healthcare advancements and increased snakebite cases.
Does ConsaInsights provide customized market report data for the antivenoms industry?
Yes, ConsaInsights offers customized market reports tailored to individual client needs, including in-depth analysis and insights specific to the antivenoms industry, ensuring relevant and actionable data.
What deliverables can I expect from this antivenoms market research project?
Delivery includes comprehensive market analysis reports, trend forecasts, regional insights, competitor analysis, and segmented data focusing on hospitals, surgical centers, and various antivenom formulations.
What are the market trends of antivenoms?
Current trends in the antivenoms market include the increasing adoption of liquid and freeze-dried formulations, a rise in partnership ventures among healthcare providers, and a focus on monovalent antivenoms for specific species.