Argatroban Market Report
Published Date: 31 January 2026 | Report Code: argatroban
Argatroban Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Argatroban market, covering detailed analysis and forecast data from 2023 to 2033. Key insights include market size, trends, competitive landscape, and regional breakdowns.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
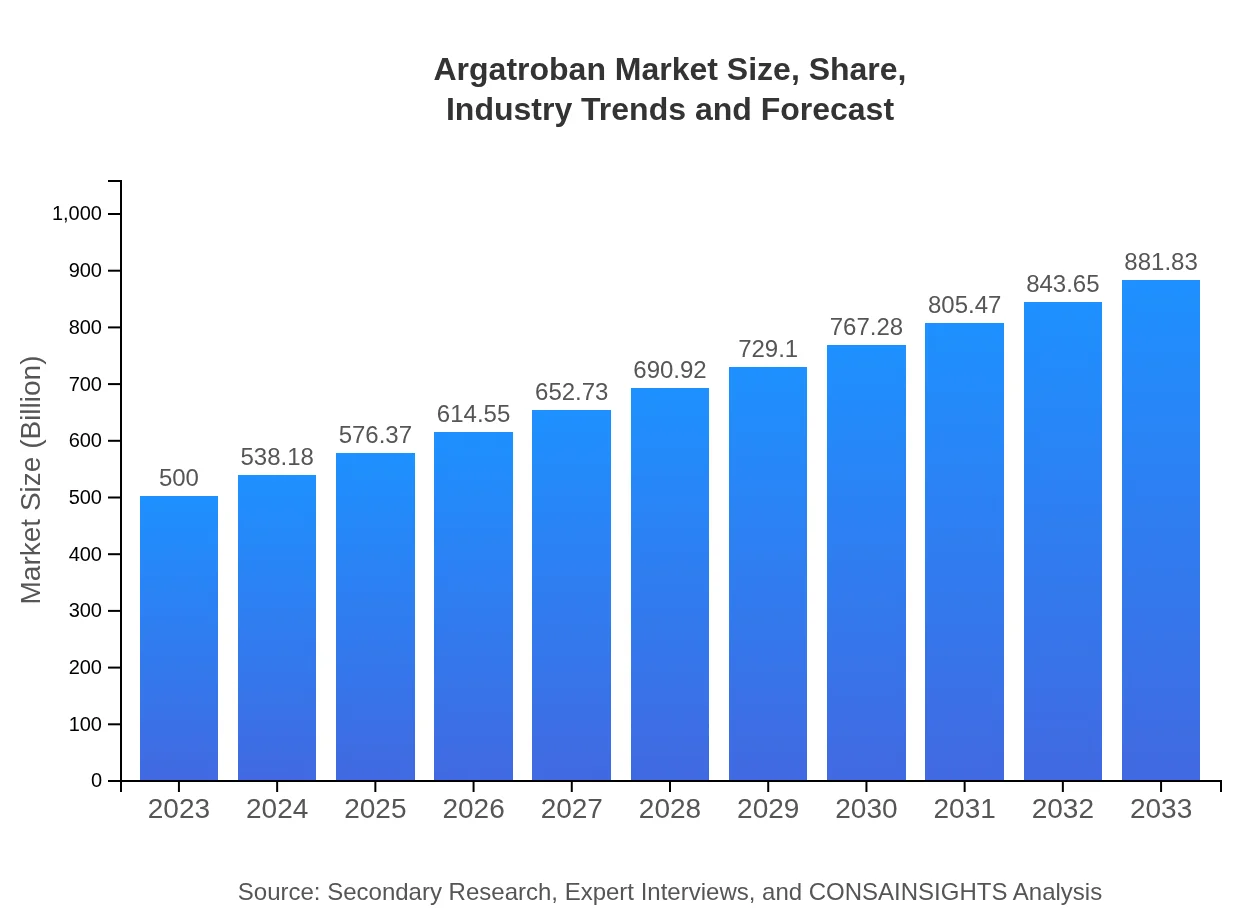
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 5.7% |
| 2033 Market Size | $881.83 Million |
| Top Companies | Baxter International, Boehringer Ingelheim, Sandoz (a Novartis division) |
| Last Modified Date | 31 January 2026 |
Argatroban Market Overview
Customize Argatroban Market Report market research report
- ✔ Get in-depth analysis of Argatroban market size, growth, and forecasts.
- ✔ Understand Argatroban's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Argatroban
What is the Market Size & CAGR of Argatroban market in 2023?
Argatroban Industry Analysis
Argatroban Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Argatroban Market Analysis Report by Region
Europe Argatroban Market Report:
The European market for Argatroban is set to witness substantial growth, increasing from $136.90 million in 2023 to $241.45 million by 2033. A strong regulatory framework, increasing adoption of advanced anticoagulants, and an aging population are key contributors to market expansion. Additionally, awareness of treatment options among healthcare providers is on the rise, promoting broader usage of Argatroban.Asia Pacific Argatroban Market Report:
In the Asia Pacific region, the Argatroban market is projected to grow from $96.70 million in 2023 to $170.55 million by 2033. Factors such as increasing healthcare expenditure, investments in medical infrastructure, and rising incidence rates of thromboembolic disorders are propelling this growth. Additionally, a growing population with a corresponding increase in chronic diseases drives demand for effective anticoagulation therapies.North America Argatroban Market Report:
North America holds a significant position in the Argatroban market, projected to grow from $165.65 million in 2023 to $292.15 million by 2033. The presence of advanced healthcare infrastructure, a higher prevalence of thromboembolic disorders, and substantial investment in research and development contribute to this growth. Additionally, the introduction of innovative product formulations is expected to enhance market sales.South America Argatroban Market Report:
South America shows a developing Argatroban market, expected to increase from $44.35 million in 2023 to $78.22 million by 2033. This growth is attributed to improving healthcare systems and public health initiatives aimed at increasing access to essential medicines. The demand for Argatroban in clinical settings is also rising as awareness of thrombosis management improves.Middle East & Africa Argatroban Market Report:
The Argatroban market in the Middle East and Africa is expected to grow from $56.40 million in 2023 to $99.47 million by 2033. Growth factors include improvements in healthcare access and an increasing number of patients needing anticoagulation beyond traditional therapy. The region’s shift towards modernized healthcare strategies and enhanced clinical practices also promotes the acceptance of innovative treatments like Argatroban.Tell us your focus area and get a customized research report.
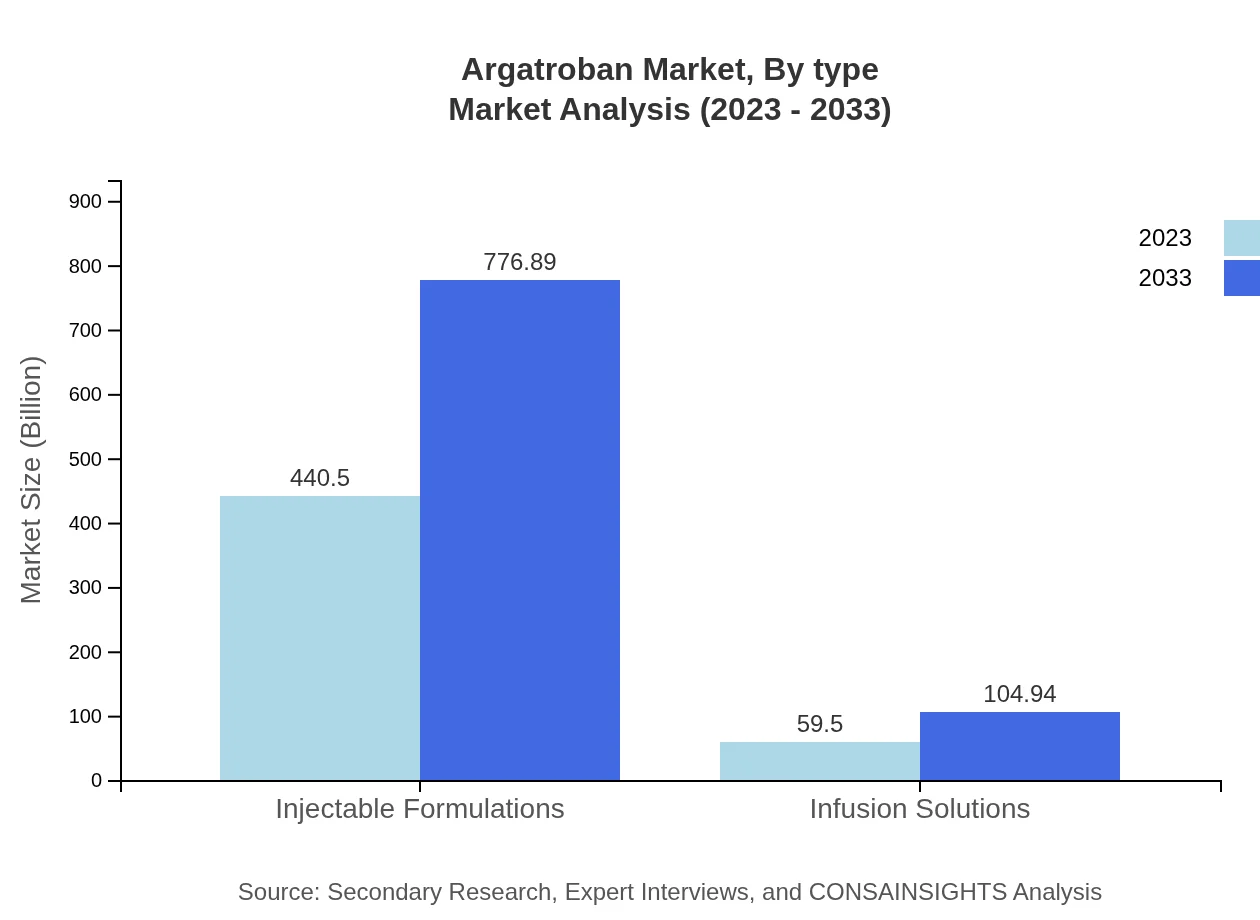
Argatroban Market Analysis By Type
The Argatroban market can be dissected based on type into Injectable Formulations and Infusion Solutions. Injectable formulations dominate the market, anticipated to grow from $440.50 million in 2023 to $776.89 million by 2033. Infusion solutions also show promise, with expectations of growth from $59.50 million to $104.94 million over the same period. These product types are essential in both acute and long-term clinical settings for effective anticoagulation management.
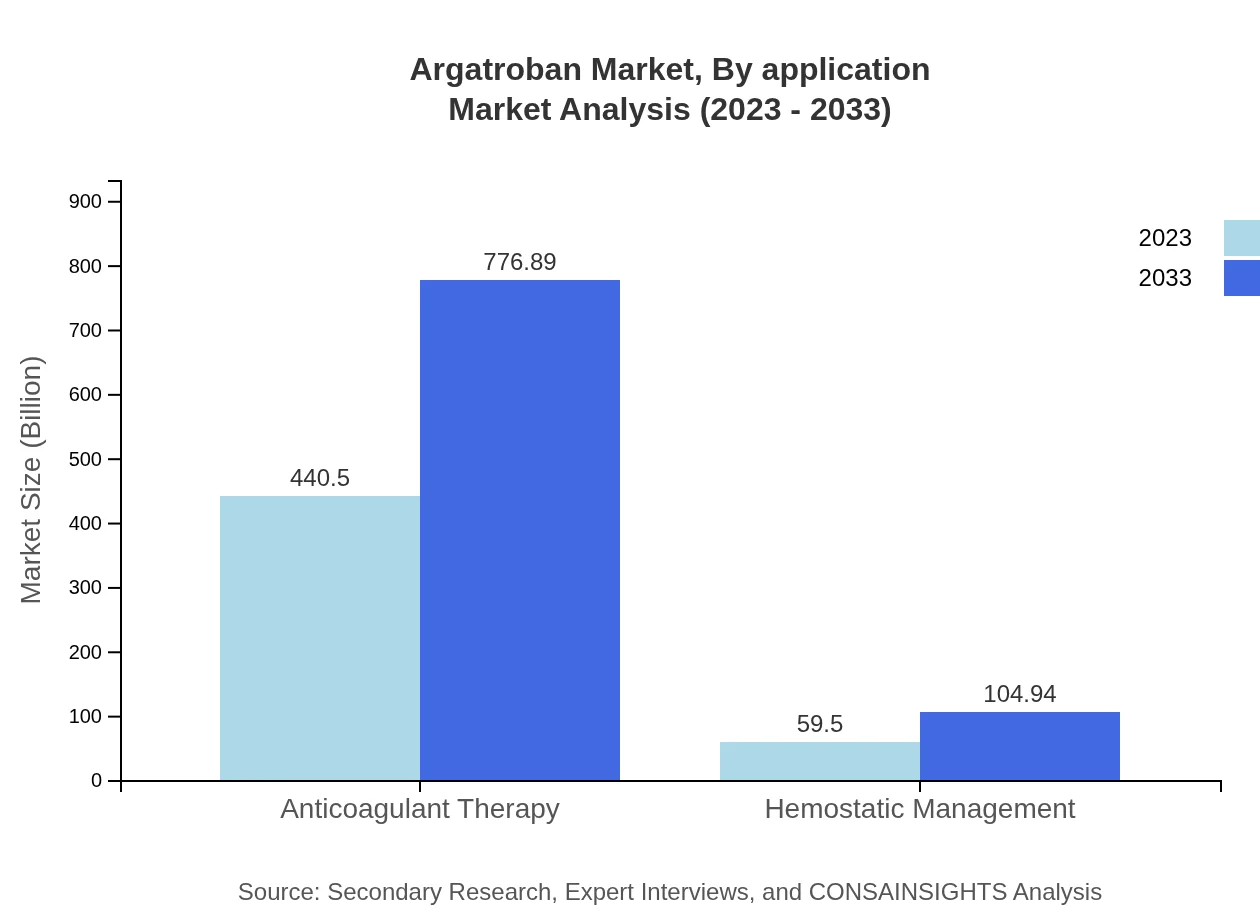
Argatroban Market Analysis By Application
Key applications for Argatroban include Anticoagulant Therapy and Hemostatic Management. Anticoagulant Therapy significantly contributes to market revenues, with an expected growth from $440.50 million to $776.89 million by 2033. Hemostatic Management is also relevant, projected to advance from $59.50 million to $104.94 million, reflecting the dual focus on preventing thrombotic events while managing potential bleeding complications.
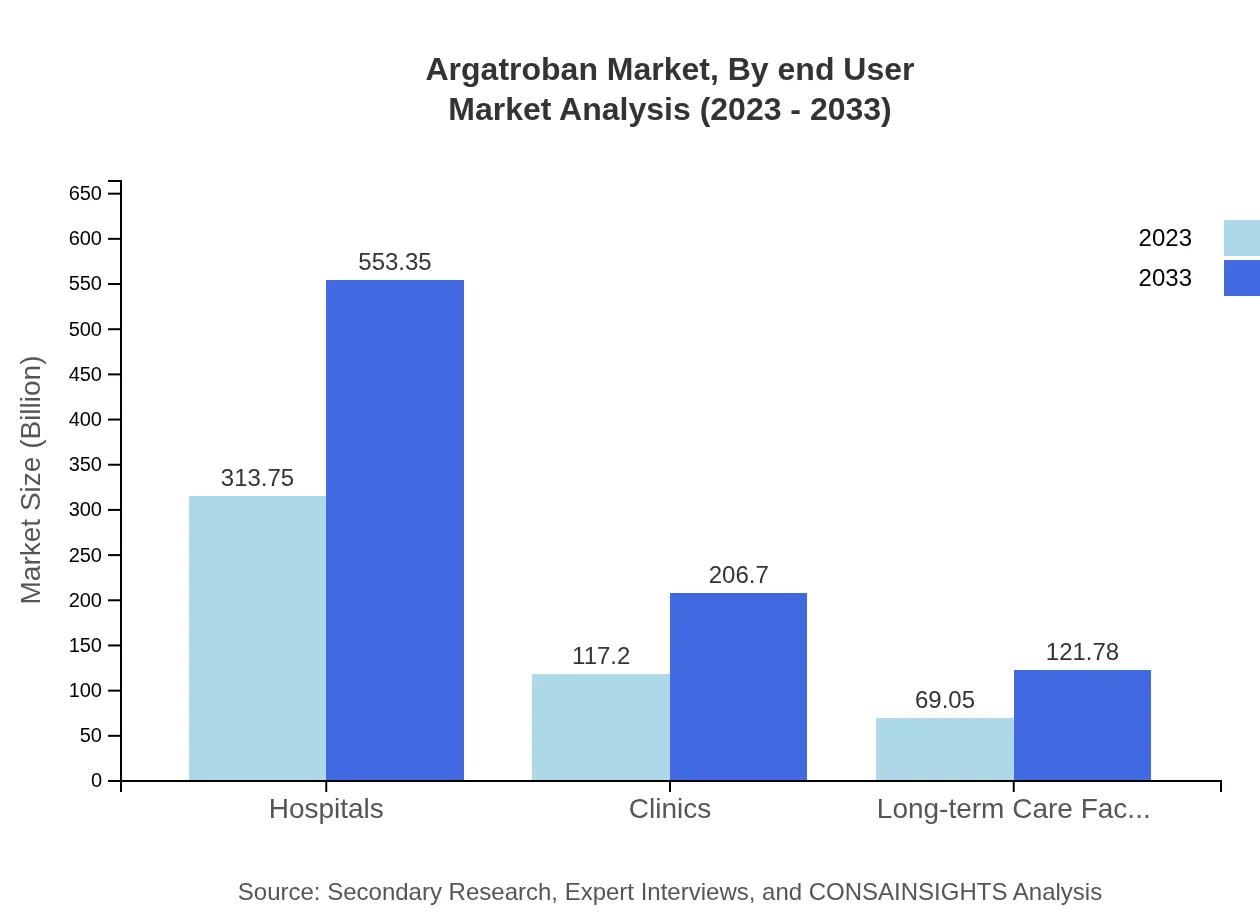
Argatroban Market Analysis By End User
End-user segmentation covers Hospitals, Clinics, Long-term Care Facilities, and Retail and Online Pharmacies. Hospitals represent the largest market share, increasing from $313.75 million to $553.35 million. Clinics are also significant, with growth from $117.20 million to $206.70 million. The evolution of healthcare delivery models and the focus on personalized care are instrumental in determining the trajectories of these segments.
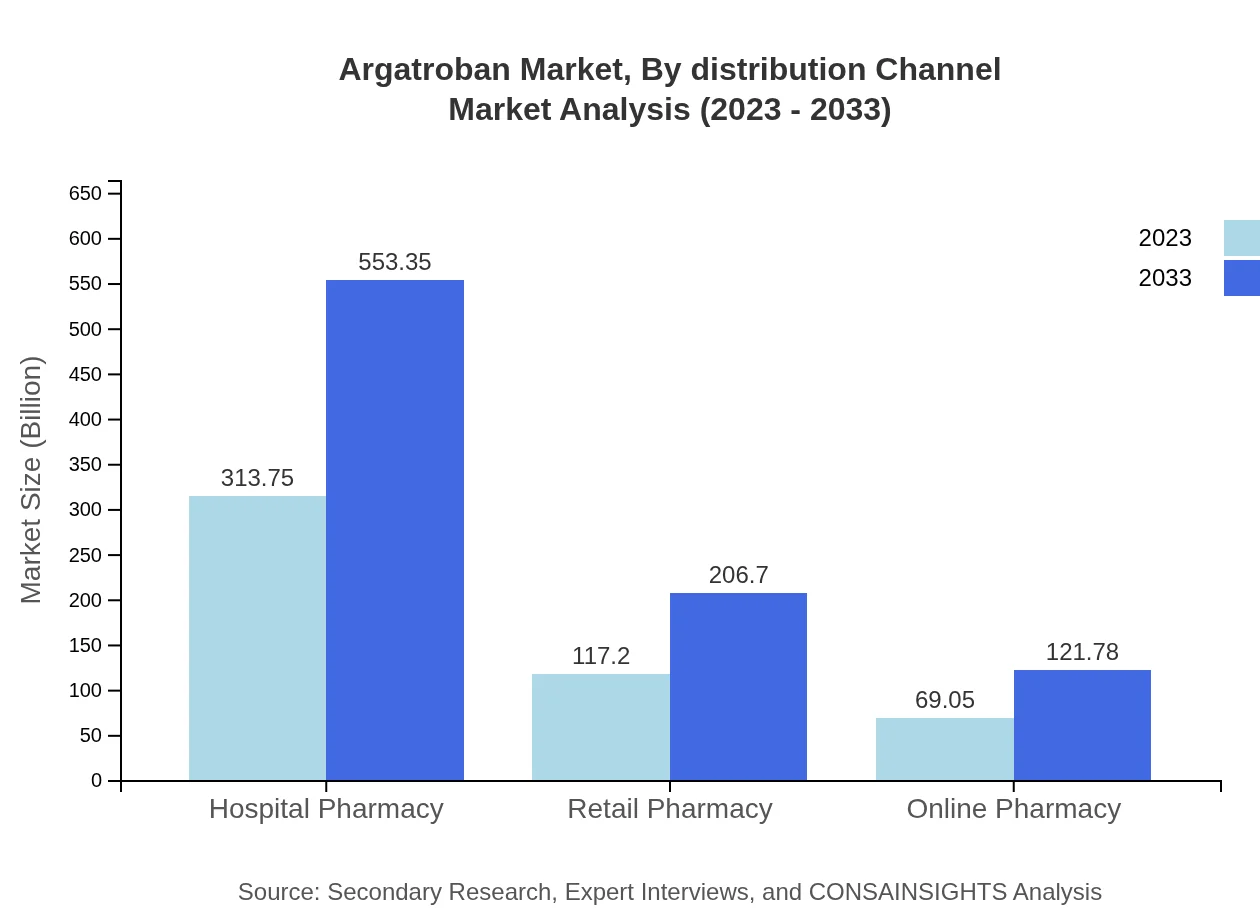
Argatroban Market Analysis By Distribution Channel
Distribution channels for Argatroban consist of Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy. Hospital Pharmacy leads the market, projected to grow from $313.75 million to $553.35 million. Retail and Online Pharmacies will further see growth, with Online Pharmacy trends reflecting an increasing shift towards e-commerce platforms in healthcare, from $69.05 million to $121.78 million.
Argatroban Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Argatroban Industry
Baxter International:
A leading biotechnology company that specializes in the development and delivery of critical therapies, Baxter is pivotal in the Argatroban market, providing innovative solutions in anticoagulant treatments.Boehringer Ingelheim:
This multinational pharmaceutical company offers a robust portfolio of anticoagulant products, including Argatroban, emphasizing research and the development of effective therapies for thromboembolic conditions.Sandoz (a Novartis division):
Sandoz develops generic pharmaceuticals, including Argatroban formulations. Their commitment to affordable biosimilars helps improve access to essential anticoagulant therapies in various regions.We're grateful to work with incredible clients.









FAQs
What is the market size of argatroban?
The argatroban market size was estimated at approximately $500 million in 2023, with a projected CAGR of 5.7% through 2033. This growth reflects increasing demand and application in anticoagulant therapies globally.
What are the key market players or companies in the argatroban industry?
Key players in the argatroban market include major pharmaceutical companies involved in anticoagulant drugs. These companies focus on innovative therapies to enhance treatment options and market reach.
What are the primary factors driving the growth in the argatroban industry?
The growth of the argatroban market is driven by rising incidences of thrombotic disorders, increased awareness about health conditions, advancements in drug formulations, and expanding healthcare infrastructure.
Which region is the fastest Growing in the argatroban market?
The fastest-growing region in the argatroban market is North America, with a market size projected to grow from $165.65 million in 2023 to $292.15 million by 2033, fueled by high healthcare expenditure.
Does ConsaInsights provide customized market report data for the argatroban industry?
Yes, ConsaInsights offers customized market report data for the argatroban industry, allowing clients to obtain tailored insights and analysis to meet their specific strategic needs.
What deliverables can I expect from this argatroban market research project?
Deliverables from the argatroban market research project include comprehensive reports with market size analysis, CAGR forecasts, competitor profiles, and regional insights tailored to your business's strategy.
What are the market trends of argatroban?
Current market trends for argatroban include a growing preference for injectable formulations, increasing usage in hospitals and clinics, and a focus on innovative anticoagulant therapies to enhance patient outcomes.