Arteriotomy Closure Devices Market Report
Published Date: 31 January 2026 | Report Code: arteriotomy-closure-devices
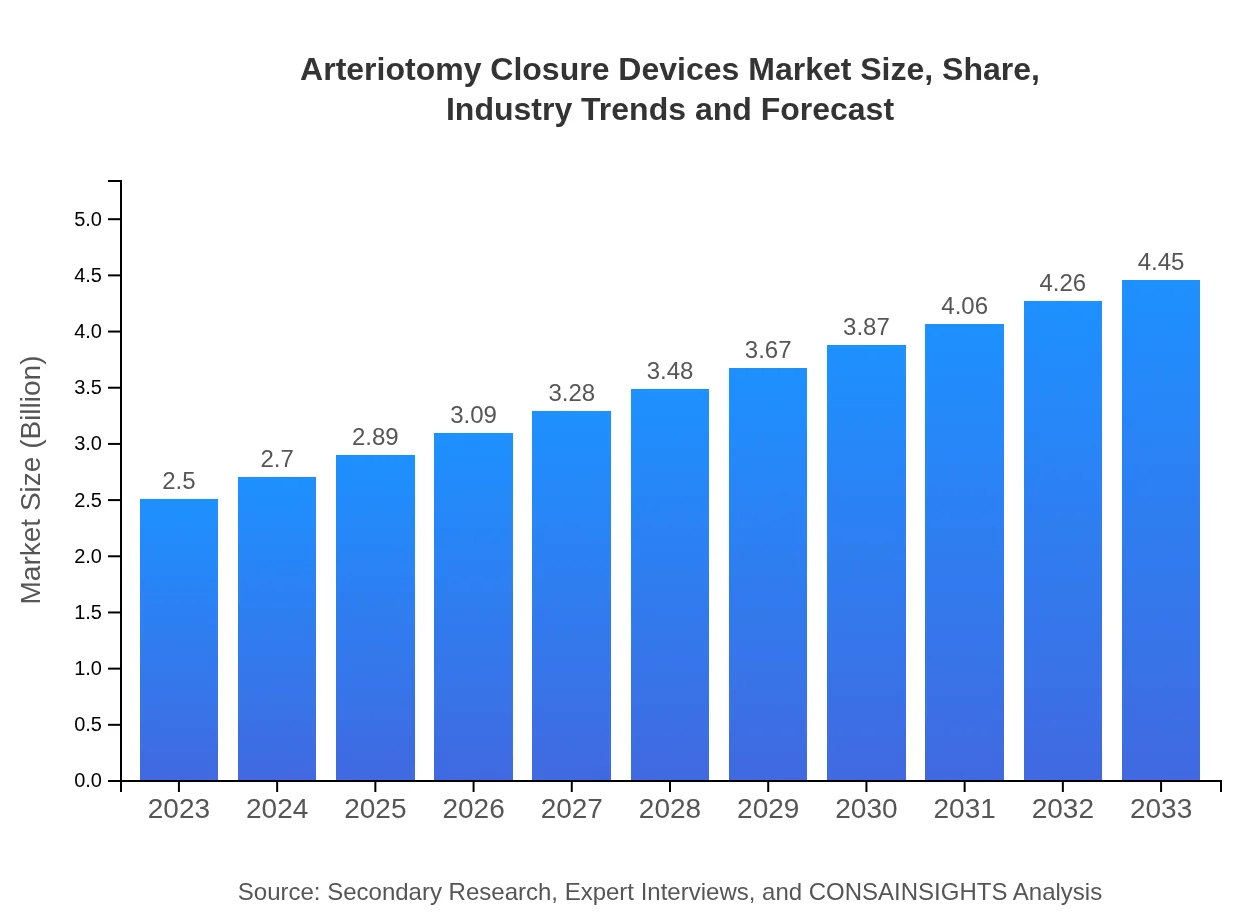
Arteriotomy Closure Devices Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Arteriotomy Closure Devices market, including current trends, growth opportunities, and detailed regional breakdowns. Insights forecast from 2023 to 2033 highlight market dynamics, key players, and future technologies shaping the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $4.45 Billion |
| Top Companies | Abbott Laboratories, Cardica, Inc., Medtronic |
| Last Modified Date | 31 January 2026 |
Arteriotomy Closure Devices Market Overview
Customize Arteriotomy Closure Devices Market Report market research report
- ✔ Get in-depth analysis of Arteriotomy Closure Devices market size, growth, and forecasts.
- ✔ Understand Arteriotomy Closure Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Arteriotomy Closure Devices
What is the Market Size & CAGR of Arteriotomy Closure Devices market in 2023?
Arteriotomy Closure Devices Industry Analysis
Arteriotomy Closure Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Arteriotomy Closure Devices Market Analysis Report by Region
Europe Arteriotomy Closure Devices Market Report:
Europe's market is projected to grow from $0.89 billion in 2023 to $1.58 billion by 2033. The region's focus on improving healthcare protocols and regulatory advancements significantly boost adoption rates of closure devices.Asia Pacific Arteriotomy Closure Devices Market Report:
The Asia Pacific region is set to witness growth, with a market size of approximately $0.43 billion in 2023, expected to reach $0.77 billion by 2033. Factors such as rising healthcare expenditures and increased prevalence of cardiovascular diseases support this growth. Countries like Japan and China exhibit high demand for advanced surgical technologies.North America Arteriotomy Closure Devices Market Report:
North America dominates the Arteriotomy Closure Devices market with a size of $0.82 billion in 2023, projected to reach $1.45 billion by 2033. Strong healthcare infrastructure and rapid technological innovations, combined with high incidences of heart disease, heavily contribute to this expansion.South America Arteriotomy Closure Devices Market Report:
In South America, the market is anticipated to grow from $0.22 billion in 2023 to about $0.39 billion by 2033. This growth is driven by increasing healthcare accessibility and the adoption of minimally invasive surgical techniques within the region.Middle East & Africa Arteriotomy Closure Devices Market Report:
The Middle East and Africa market is smaller but growing, with a size of $0.15 billion expected to rise to $0.26 billion by 2033. This growth is largely influenced by enhanced healthcare services in urban areas and investment in healthcare infrastructure.Tell us your focus area and get a customized research report.
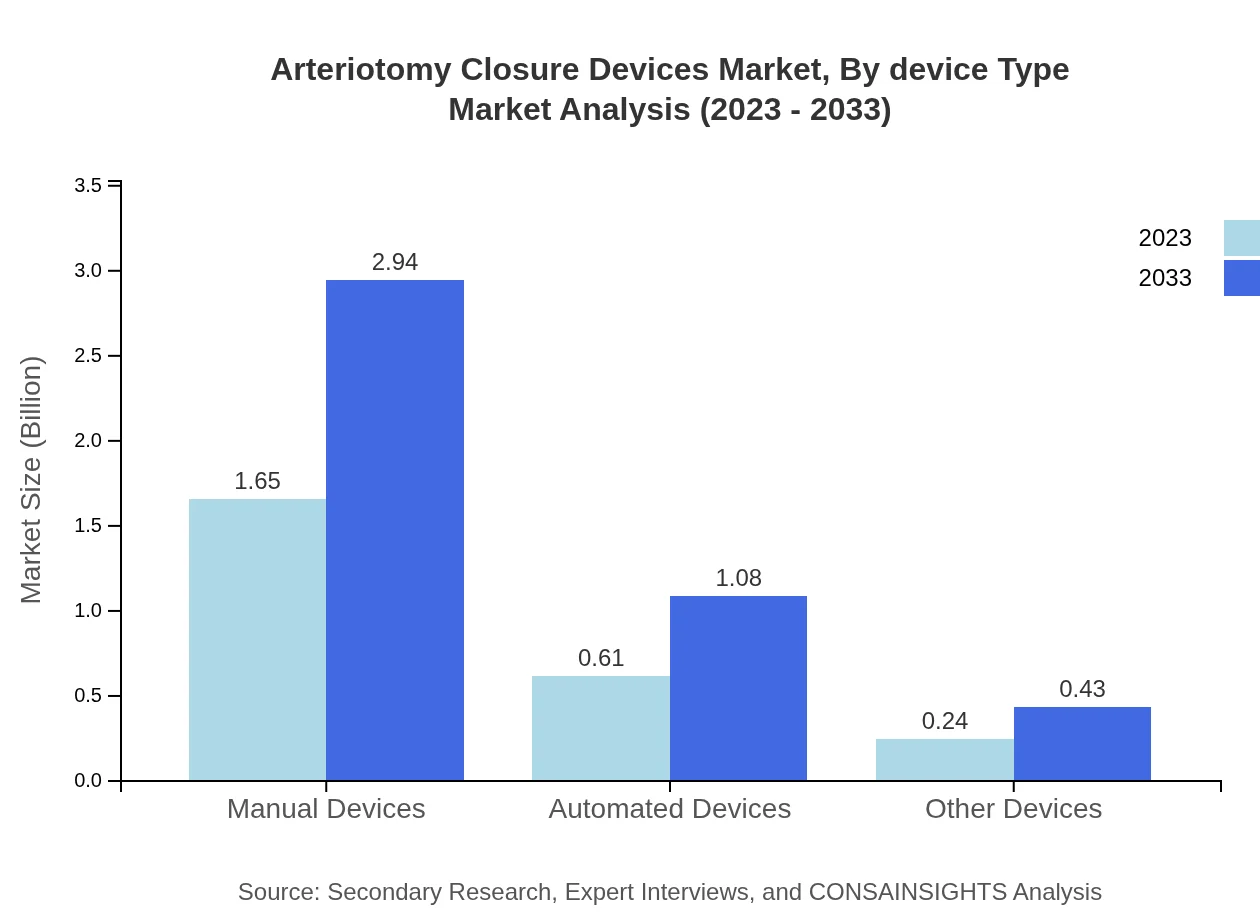
Arteriotomy Closure Devices Market Analysis By Device Type
The market for Arteriotomy Closure Devices is categorized into manual, automated, and other devices. Manual devices hold a significant share of around 66% in 2023, expected to maintain this share through 2033, reflecting their established efficacy and adoption in surgical environments. Automated devices are also gaining traction, projected to grow from $0.61 billion in 2023 to $1.08 billion by 2033, driven by advancements in automation and robotic-assisted surgeries.
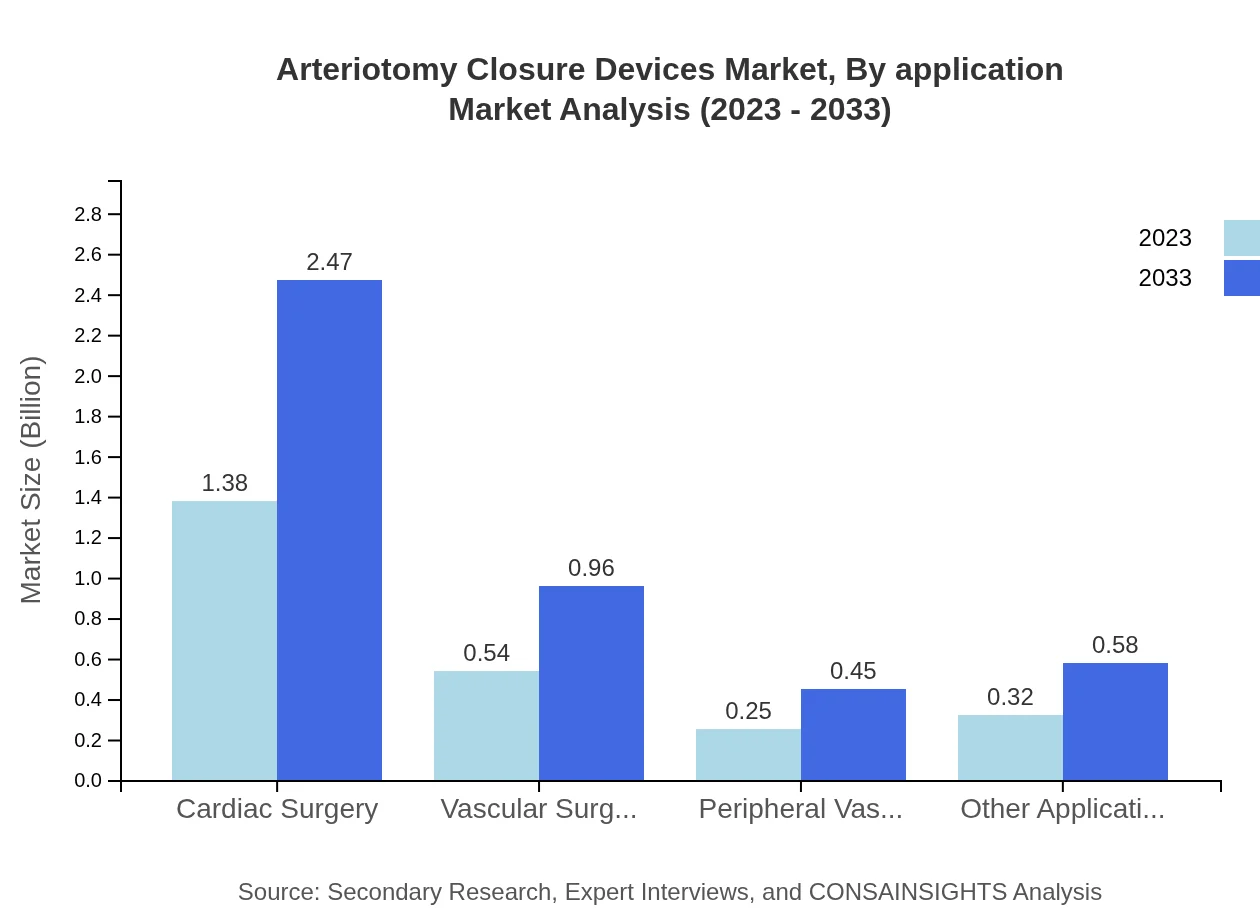
Arteriotomy Closure Devices Market Analysis By Application
Applications of Arteriotomy Closure Devices include cardiac surgery, vascular surgery, peripheral vascular interventions, and other procedures. Cardiac surgeries dominate with around 55% of the market in 2023, expanding due to the increasing number of cardiovascular operations. Vascular surgeries are also significant, anticipated to grow slowly but steadily as the need for vascular interventions rises.
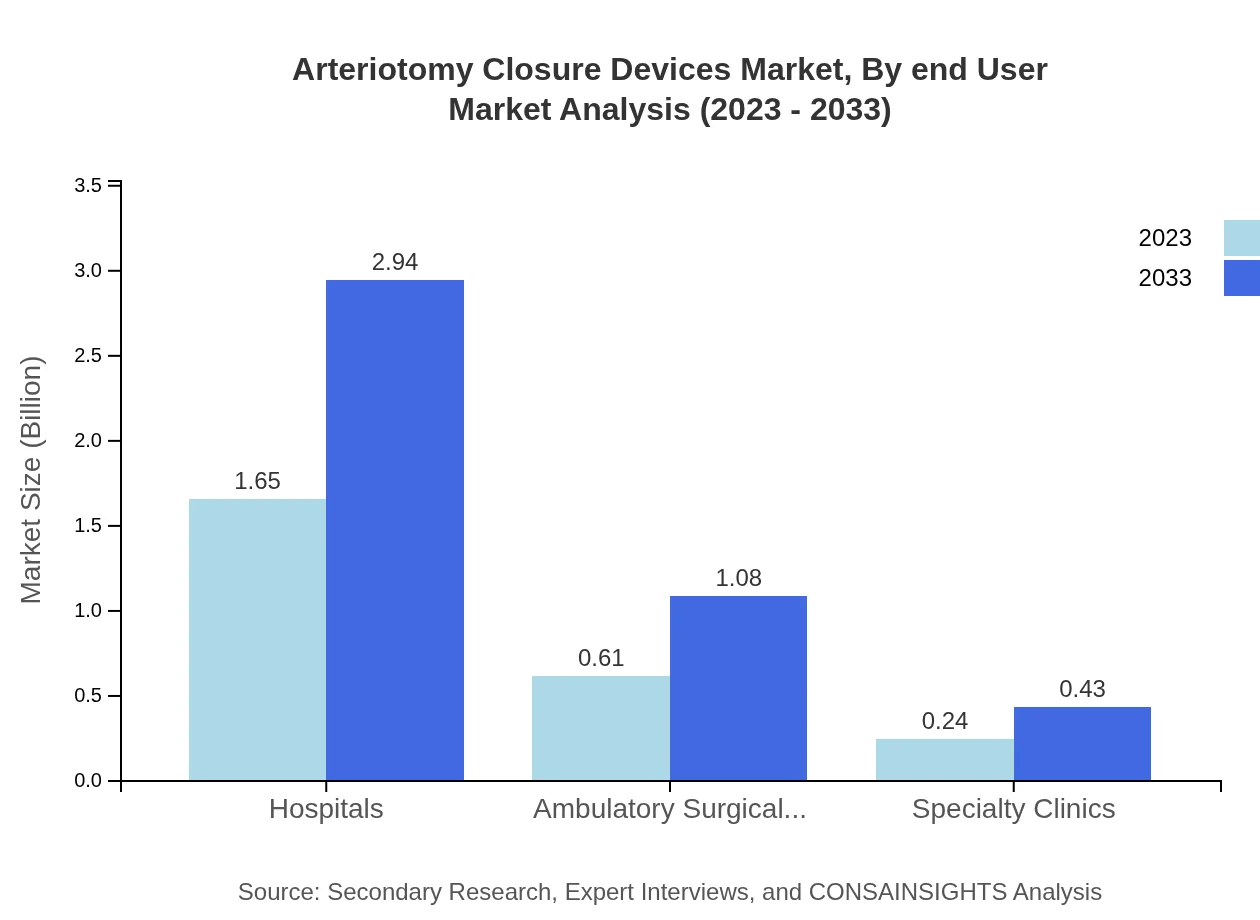
Arteriotomy Closure Devices Market Analysis By End User
Hospitals remain the predominant end-user, with a market share of approximately 66.08% in 2023, ensuring continued growth as more complex procedures are performed. Ambulatory surgical centers are growing rapidly, projected to double their market share by 2033, reflecting a trend toward outpatient care.
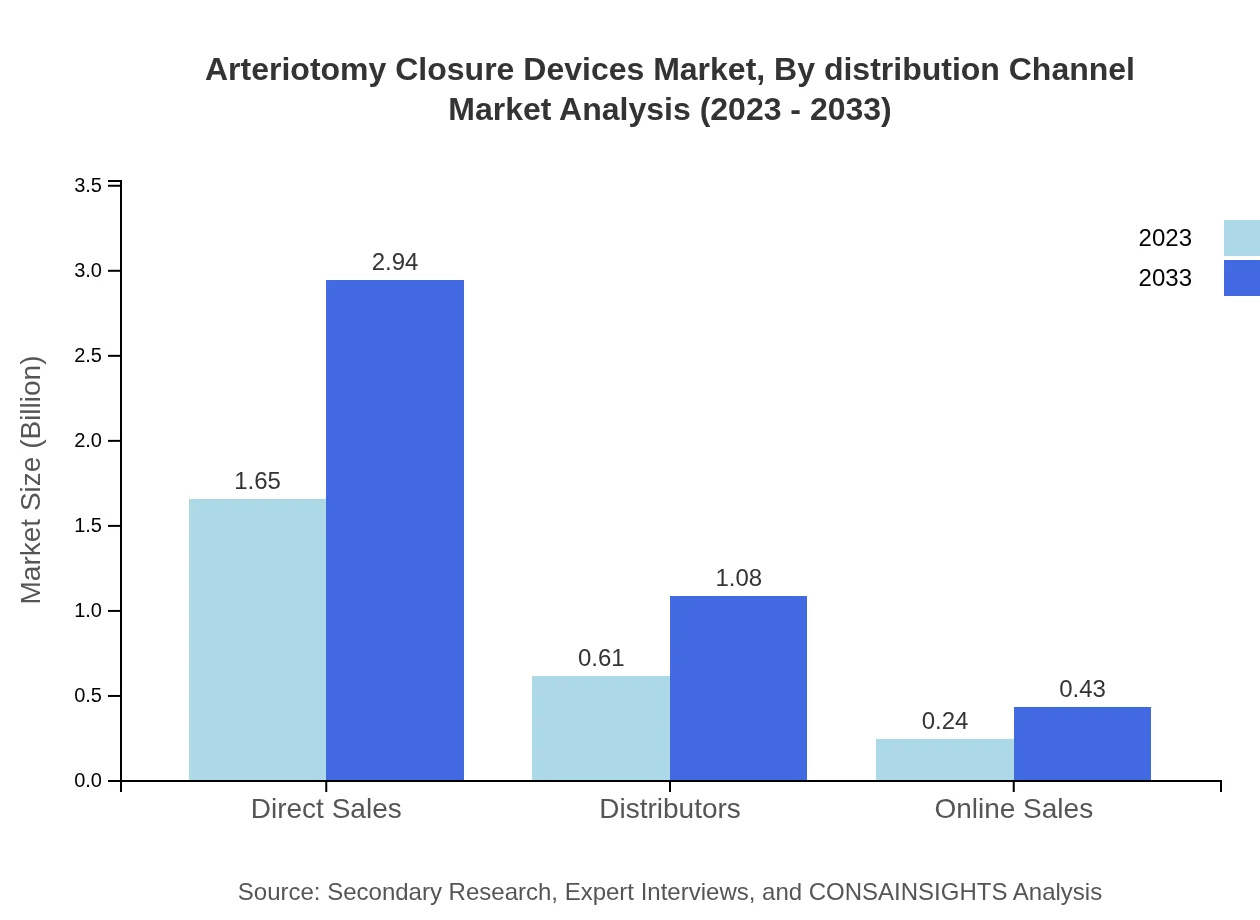
Arteriotomy Closure Devices Market Analysis By Distribution Channel
Distribution channels for Arteriotomy Closure Devices include direct sales, distributors, and online sales. Direct sales dominate with a 66.08% share in 2023 due to established purchasing relationships with hospitals, while online sales are expected to increase in importance as e-commerce in healthcare expands.
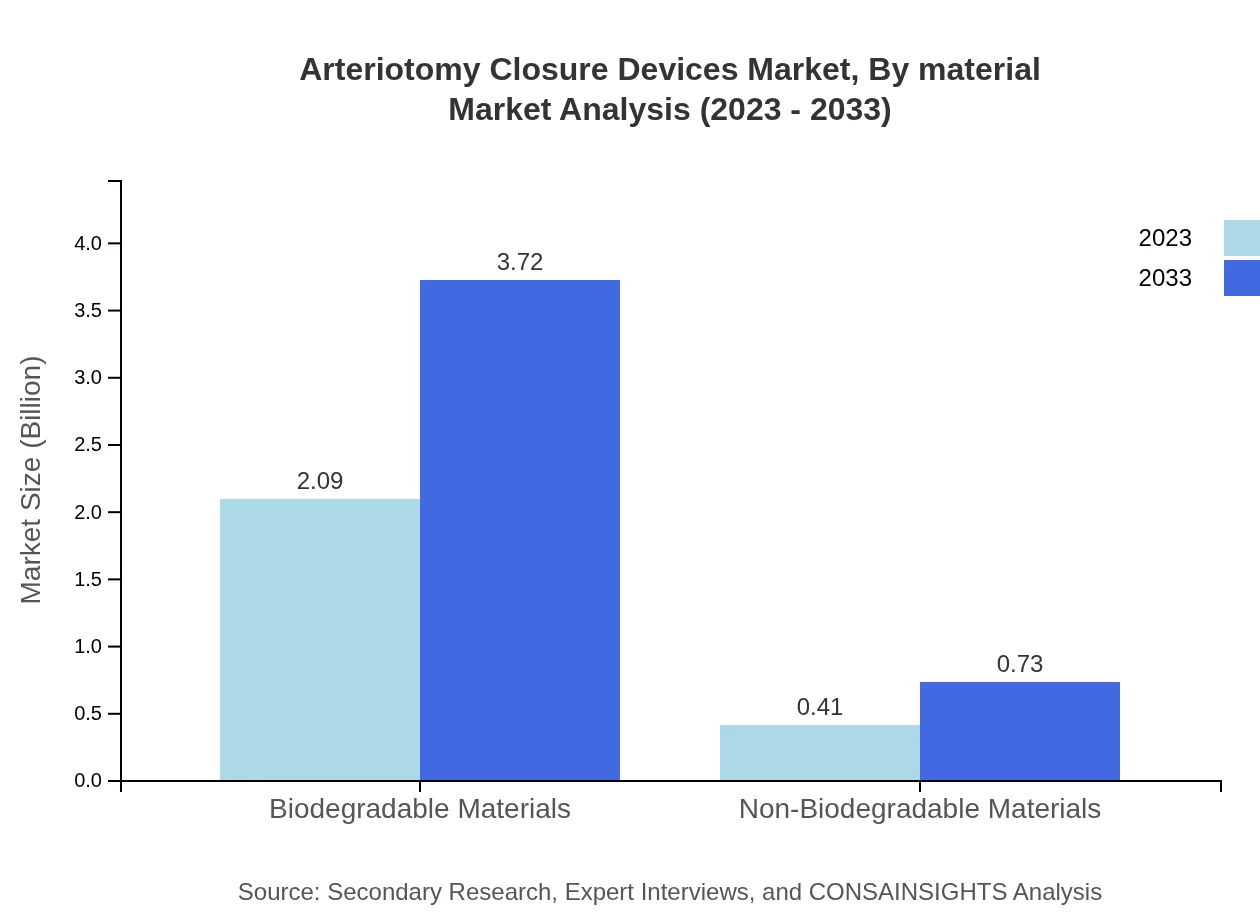
Arteriotomy Closure Devices Market Analysis By Material
Materials used in Arteriotomy Closure Devices can be divided into biodegradable and non-biodegradable. Biodegradable materials capture a significant market share of 83.58%, driven by their benefits in preventing complications associated with foreign materials. Further advances in materials science are likely to enhance this segment's dominance in the coming years.
Arteriotomy Closure Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Arteriotomy Closure Devices Industry
Abbott Laboratories:
A leader in medical devices, Abbott specializes in advanced cardiovascular solutions, including innovative arteriotomy closure devices that enhance surgical outcomes.Cardica, Inc.:
Cardica is known for its innovative closure technologies and devices designed to streamline surgical procedures and improve recovery times for patients undergoing arterial interventions.Medtronic :
One of the largest medical device companies globally, Medtronic offers a comprehensive range of surgical products, including cutting-edge arteriotomy closure devices widely used in cardiac surgeries.We're grateful to work with incredible clients.









FAQs
What is the market size of arteriotomy Closure Devices?
The global arteriotomy-closure devices market is valued at approximately $2.5 billion in 2023. It is projected to grow at a CAGR of 5.8%, aspiring to reach significant growth over the next decade, with continuous innovations and increasing patient volumes.
What are the key market players or companies in this arteriotomy Closure Devices industry?
Key players in the arteriotomy-closure devices market include notable companies like Abbott Laboratories, Medtronic, and Terumo Corporation. Their contributions to innovation and technology advancements play a crucial role in the market dynamics and competitive landscape.
What are the primary factors driving the growth in the arteriotomy Closure Devices industry?
The growth of the arteriotomy-closure devices market is propelled by the rising demand for minimally invasive surgeries, technological advancements, and an increasing number of cardiovascular procedures. Additionally, an aging population and heightened awareness of procedural safety contribute significantly.
Which region is the fastest Growing in the arteriotomy Closure Devices?
North America is identified as the fastest-growing region in the arteriotomy-closure devices market, with an estimated market size of $1.45 billion by 2033, driven by advanced healthcare infrastructure, increased adoption of new technologies, and a higher prevalence of cardiovascular diseases.
Does ConsaInsights provide customized market report data for the arteriotomy Closure Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the arteriotomy-closure devices industry. Clients can receive detailed insights and analytics suitable for strategic decision-making.
What deliverables can I expect from this arteriotomy Closure Devices market research project?
Deliverables from the arteriotomy-closure devices market research project include comprehensive reports, analytical insights, market forecasts, regional data analysis, segmentation breakdown, and competitive landscape assessments to support informed decision-making.
What are the market trends of arteriotomy Closure Devices?
Current market trends for arteriotomy-closure devices showcase an emphasis on biodegradable materials, increasing automation in production processes, and evolving surgical techniques focused on minimizing risks and enhancing patient outcomes, particularly in cardiovascular surgeries.