Artificial Blood Vessels Market Report
Published Date: 31 January 2026 | Report Code: artificial-blood-vessels
Artificial Blood Vessels Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Artificial Blood Vessels market, including growth trends, segmentation, and regional dynamics from 2023 to 2033. It offers insights into market size, key players, and future forecasts to guide stakeholders in decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
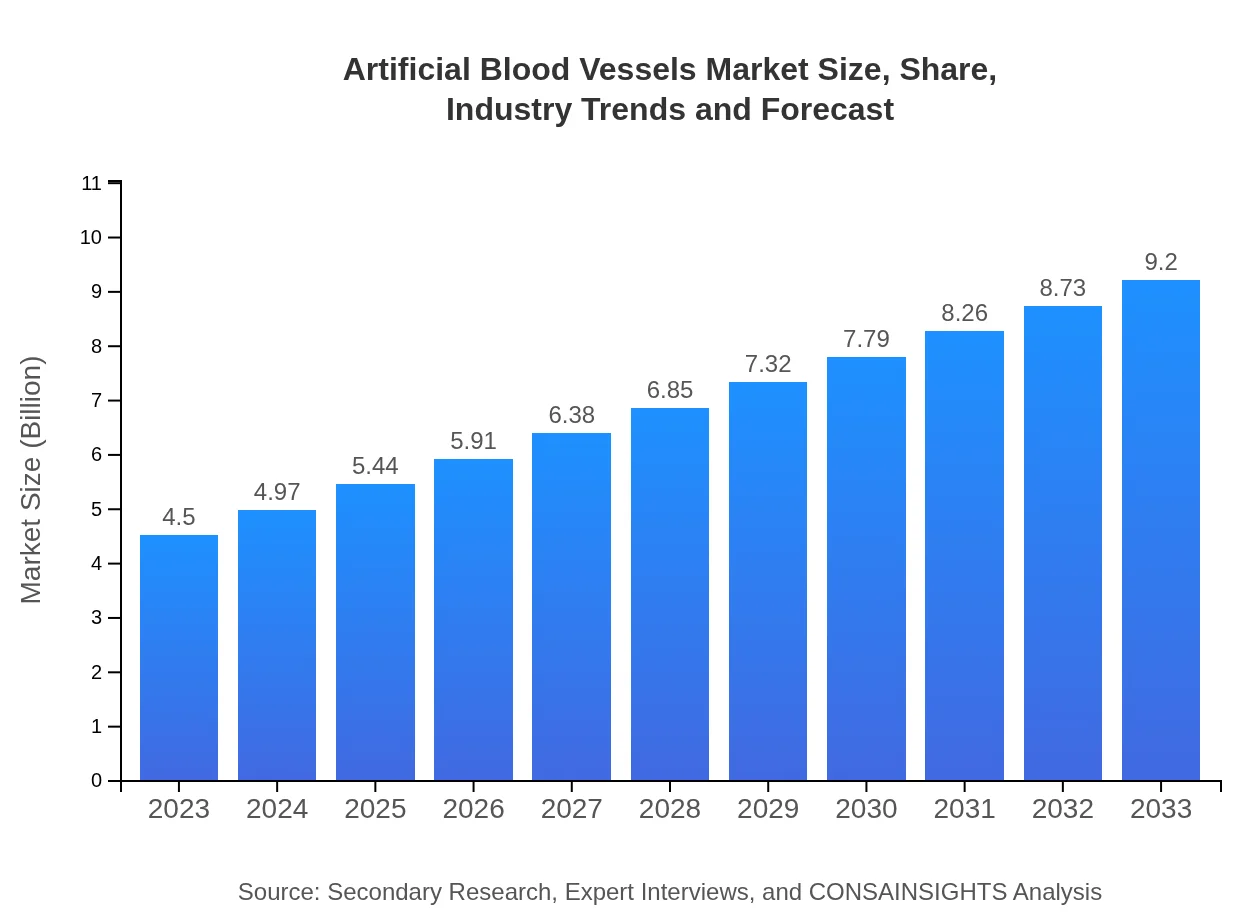
| 2023 Market Size | $4.50 Billion |
| CAGR (2023-2033) | 7.2% |
| 2033 Market Size | $9.20 Billion |
| Top Companies | Medtronic , Boston Scientific, Terumo Corporation, CryoLife, Inc. |
| Last Modified Date | 31 January 2026 |
Artificial Blood Vessels Market Overview
Customize Artificial Blood Vessels Market Report market research report
- ✔ Get in-depth analysis of Artificial Blood Vessels market size, growth, and forecasts.
- ✔ Understand Artificial Blood Vessels's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Artificial Blood Vessels
What is the Market Size & CAGR of Artificial Blood Vessels market in 2023?
Artificial Blood Vessels Industry Analysis
Artificial Blood Vessels Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Artificial Blood Vessels Market Analysis Report by Region
Europe Artificial Blood Vessels Market Report:
The European market, beginning at USD 1.42 billion in 2023 and projected at USD 2.90 billion by 2033, signifies strong growth backed by a supportive regulatory environment and rising healthcare costs. An increasing focus on personalized medicine drives innovation in artificial vascular grafts across various European countries.Asia Pacific Artificial Blood Vessels Market Report:
In 2023, the Asia Pacific region holds a market size of USD 0.80 billion and is projected to grow to USD 1.63 billion by 2033. The increasing prevalence of cardiovascular diseases and a growing elderly population are primary drivers of this growth. Investments in healthcare infrastructure and technological advancements further enhance market prospects.North America Artificial Blood Vessels Market Report:
North America is a dominant region in the Artificial Blood Vessels market, starting at USD 1.71 billion in 2023 and estimated to see growth to USD 3.49 billion by 2033. High healthcare expenditure, advanced medical technologies, and the presence of major market players contribute to this lead. The region's robust research and clinical trials further cement its position.South America Artificial Blood Vessels Market Report:
The South American market for Artificial Blood Vessels is relatively smaller, with a size of USD 0.15 billion in 2023, anticipated to reach USD 0.31 billion by 2033. Limited access to advanced medical technologies and economic challenges currently restrain market growth. However, ongoing health initiatives and the rise in healthcare access are expected to boost demand gradually.Middle East & Africa Artificial Blood Vessels Market Report:
The Middle East and Africa market is valued at USD 0.42 billion in 2023 and is expected to grow modestly to USD 0.87 billion by 2033. The primary growth drivers include increasing awareness of vascular diseases and improving healthcare facilities. However, regional disparities in healthcare access remain a challenge.Tell us your focus area and get a customized research report.
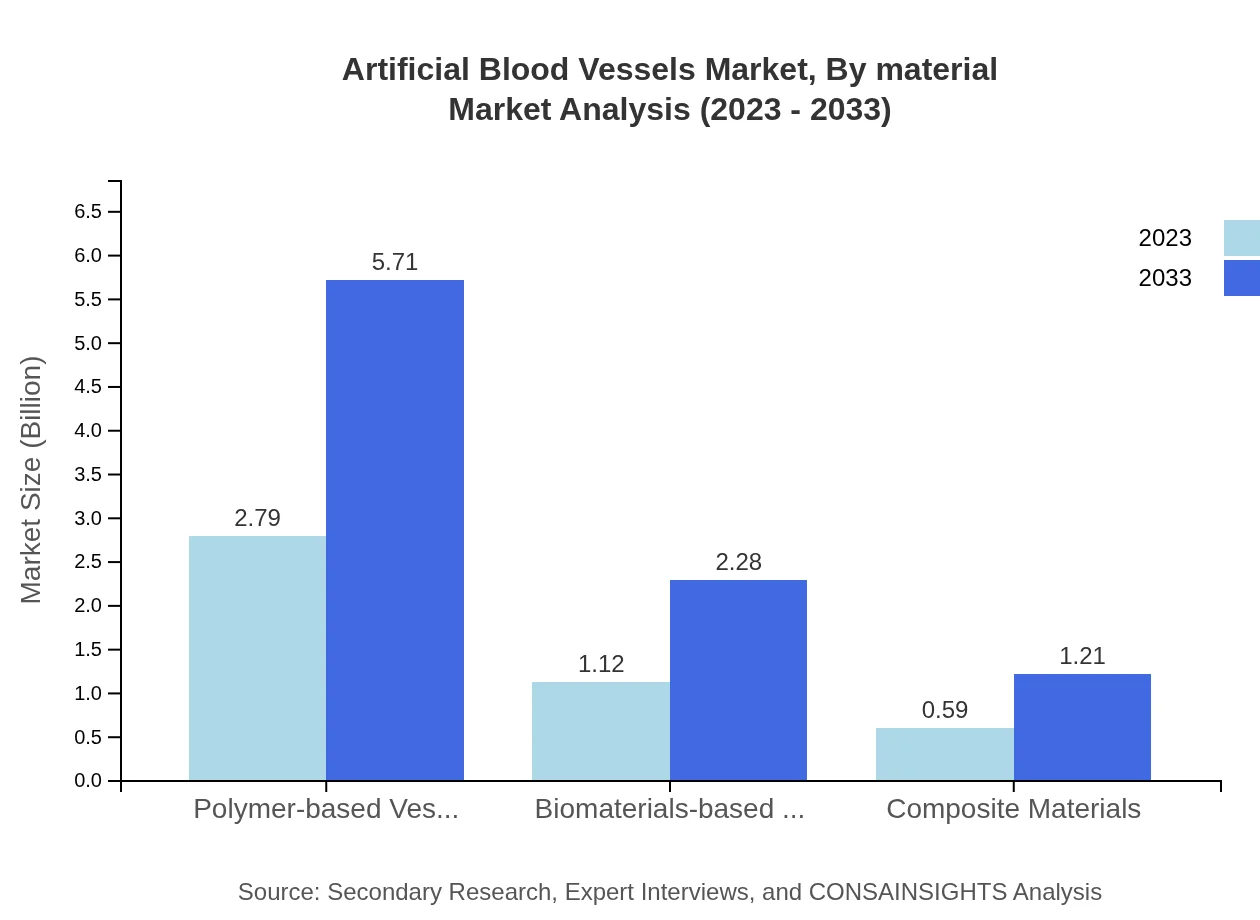
Artificial Blood Vessels Market Analysis By Material
The market, segmented by material, features polymer-based vessels holding the largest share. In 2023, polymer-based vessels command a market size of USD 2.79 billion, projected to reach USD 5.71 billion by 2033, maintaining a steady market share of 62.09%. Biomaterials-based vessels and composite materials follow, with market sizes of USD 1.12 billion and USD 0.59 billion respectively, indicating their significance and potential for growth.
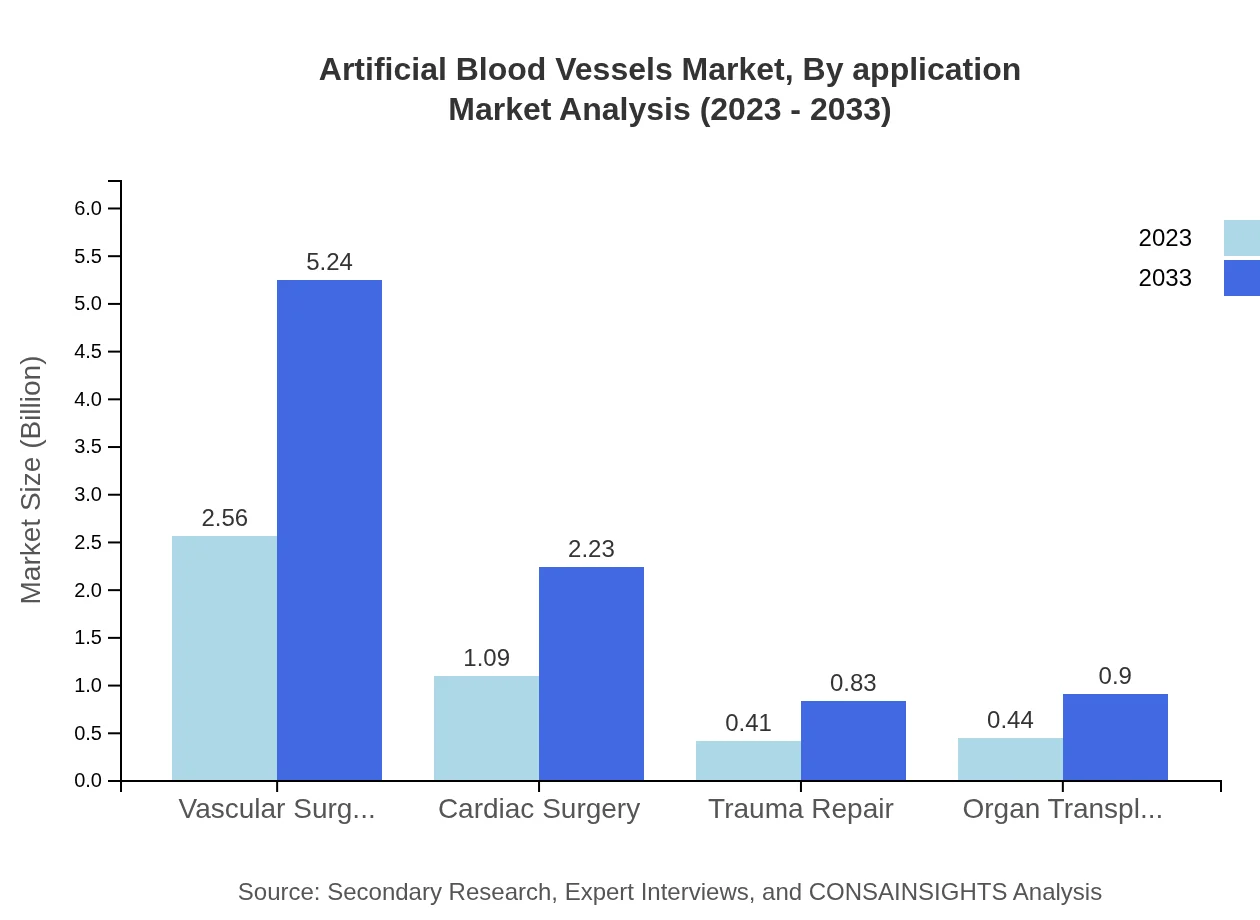
Artificial Blood Vessels Market Analysis By Application
Applications for artificial blood vessels span vascular surgeries, cardiac surgeries, trauma repair, and organ transplantation. Vascular surgeries are expected to dominate with a market size of USD 2.56 billion in 2023 and projected to be USD 5.24 billion by 2033. Cardiac surgeries will also show substantial growth from USD 1.09 billion to USD 2.23 billion during the same period, reflecting increased surgical procedures.
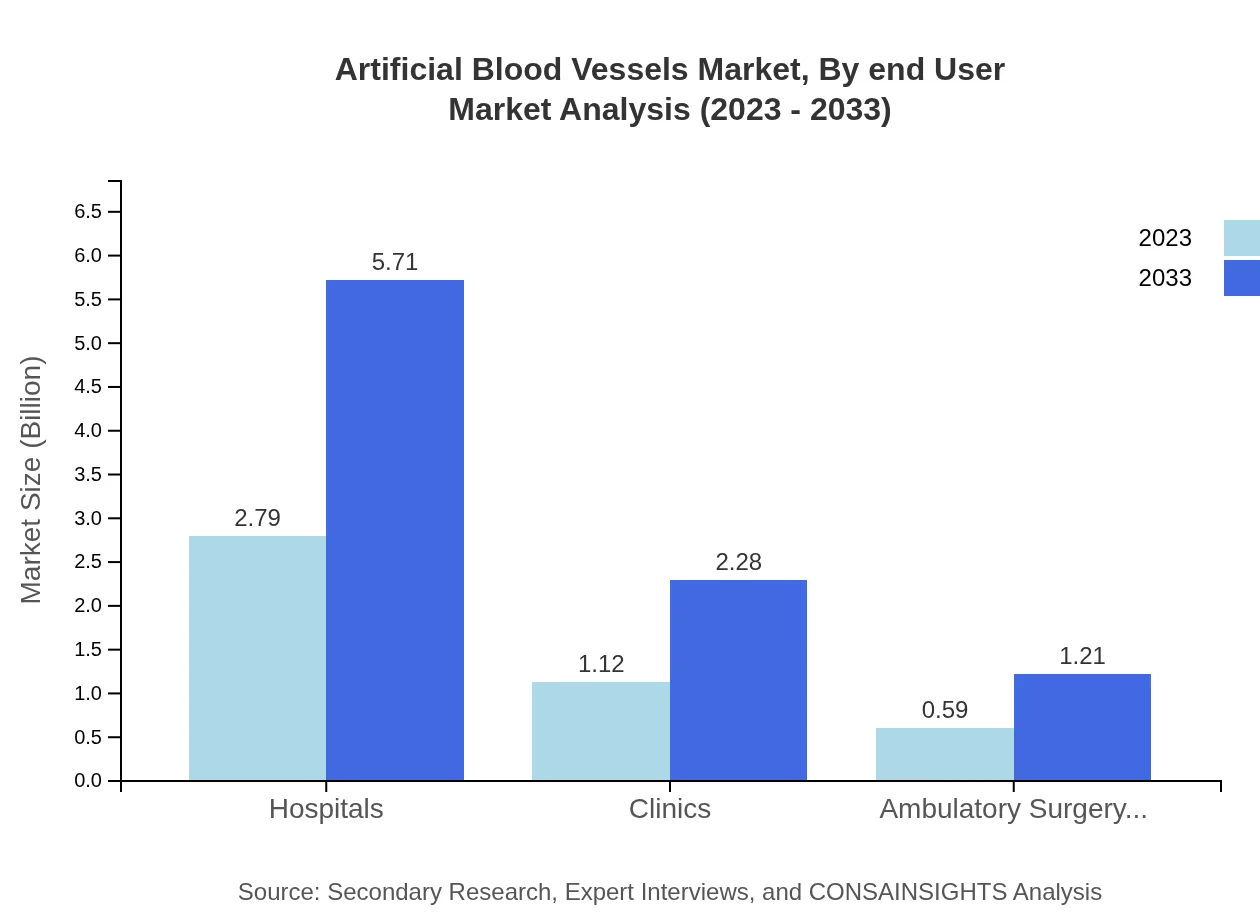
Artificial Blood Vessels Market Analysis By End User
In terms of end-users, hospitals remain the primary consumers of artificial blood vessels with a market size of USD 2.79 billion in 2023. This segment is anticipated to reach USD 5.71 billion by 2033. Clinics and ambulatory surgery centers also play crucial roles, accounting for USD 1.12 billion and USD 0.59 billion respectively in 2023.
Artificial Blood Vessels Market Analysis By Region
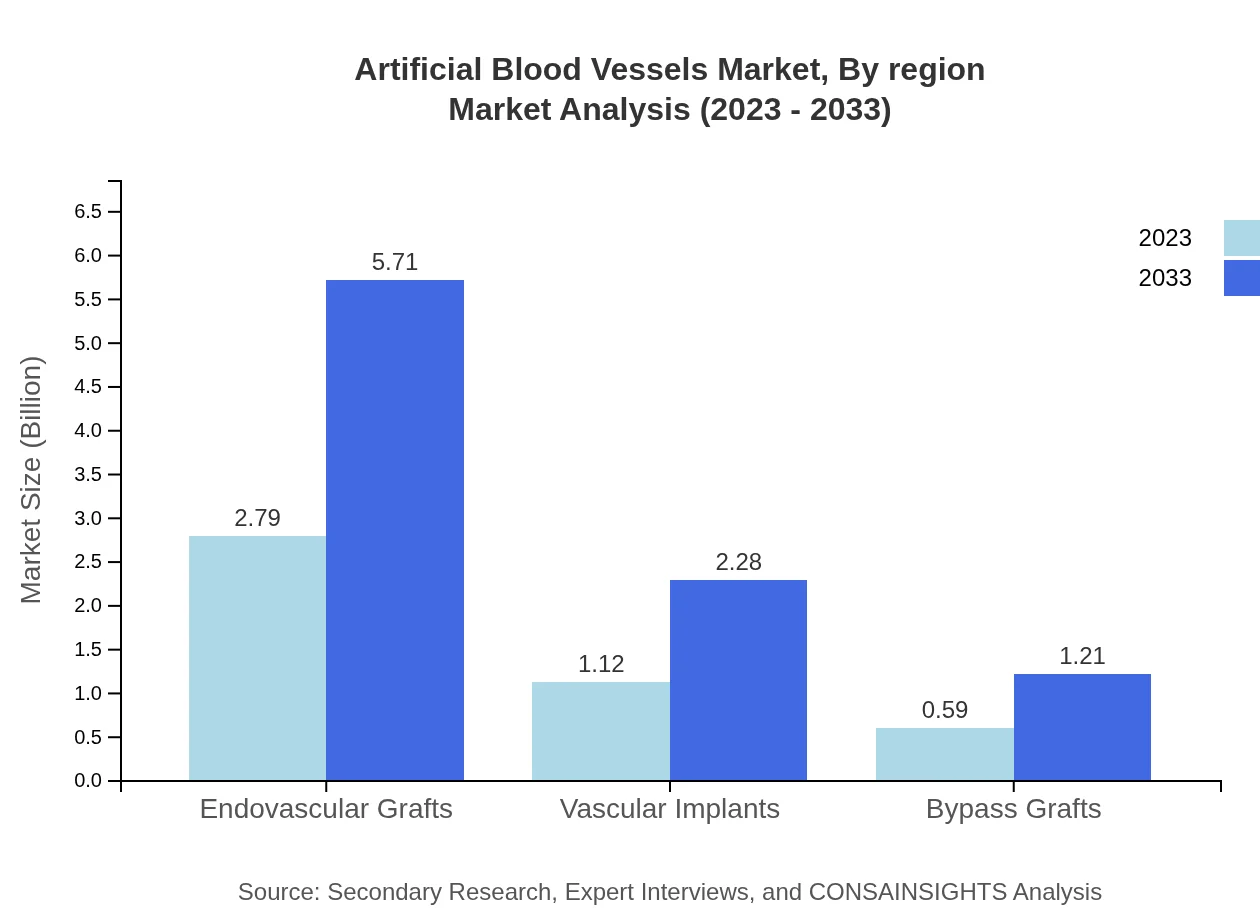
The product type analysis includes endovascular grafts, vascular implants, and bypass grafts. Endovascular grafts dominate the market with a size of USD 2.79 billion in 2023, expected to grow to USD 5.71 billion by 2033, capturing a 62.09% market share. Conversely, bypass grafts showcase a modest growth trajectory, starting from USD 0.59 billion and enhancing to USD 1.21 billion.
Artificial Blood Vessels Market Analysis By Distribution Channel
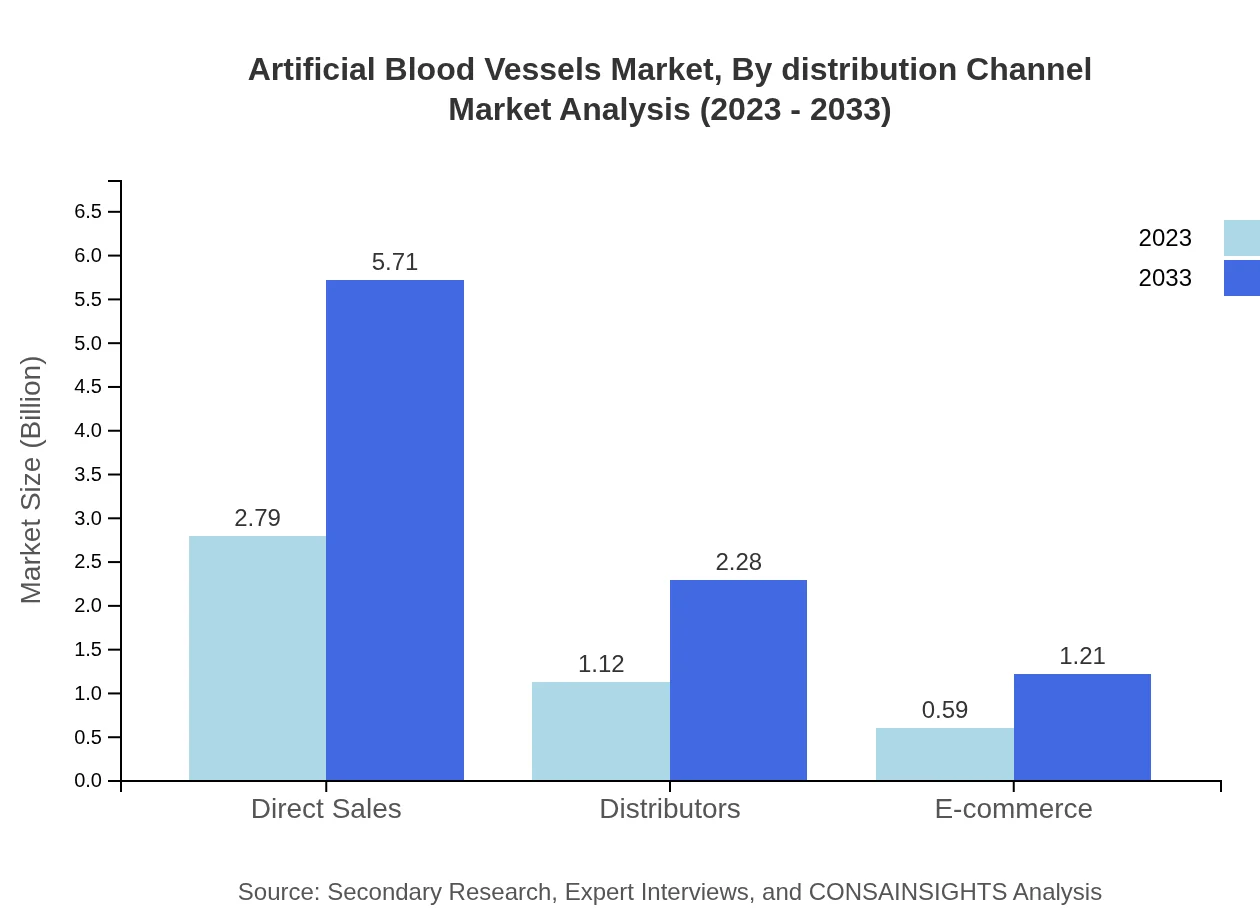
Distribution channels for artificial blood vessels include direct sales, distributors, and e-commerce. Direct sales are leading with a market size of USD 2.79 billion in 2023, projected to reach USD 5.71 billion by 2033. Distributors will also showcase significant growth, increasing from USD 1.12 billion to USD 2.28 billion.
Artificial Blood Vessels Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Artificial Blood Vessels Industry
Medtronic :
Medtronic is a leading player in the global medical technology arena, particularly known for its artificial blood vessels and vascular grafts that are widely used in various surgical procedures.Boston Scientific:
Boston Scientific develops innovative medical solutions, including state-of-the-art vascular grafts that support patient recovery and outcomes, enhancing their position in the artificial blood vessels market.Terumo Corporation:
Terumo focuses on the high-quality production of medical devices, including artificial blood vessels, emphasizing research and development to ensure cutting-edge solutions.CryoLife, Inc.:
CryoLife is recognized for its advanced preservation technologies for tissues and vascular grafts, leading innovative approaches in the artificial blood vessels segment.We're grateful to work with incredible clients.









FAQs
What is the market size of artificial blood vessels?
The global market size for artificial blood vessels is estimated at $4.5 billion as of 2023, with a projected CAGR of 7.2% from 2023 to 2033, indicating significant growth opportunities in this sector.
What are the key market players or companies in the artificial blood vessels industry?
Key players in the artificial blood vessels market include established medical device manufacturers, biotechnology firms, and innovative startups focused on vascular technologies. These companies invest heavily in R&D to advance the field and maintain competitive advantages.
What are the primary factors driving the growth in the artificial blood vessels industry?
Growth in the artificial blood vessels industry is primarily driven by rising incidences of vascular diseases, technological advancements in biomaterials, increasing geriatric population, and expanding healthcare infrastructure, which collectively enhance demand for vascular solutions.
Which region is the fastest Growing in the artificial blood vessels market?
North America is the fastest-growing region in the artificial blood vessels market. The market size in North America is projected to grow from $1.71 billion in 2023 to $3.49 billion by 2033, reflecting an increasing investment in healthcare technologies.
Does ConsaInsights provide customized market report data for the artificial blood vessels industry?
Yes, ConsaInsights offers customized market report data tailored to client needs in the artificial blood vessels industry. This includes specific analyses by region, segment, and market trends to help clients make informed decisions.
What deliverables can I expect from this artificial blood vessels market research project?
Deliverables from this market research project include comprehensive reports on market size, growth forecasts, competitive landscape analysis, regional insights, and detailed segment breakdowns, which are essential for strategic planning.
What are the market trends of artificial blood vessels?
Current trends in the artificial blood vessels market include a shift towards polymer-based vessels, increasing adoption of minimally invasive surgical techniques, growth in endovascular graft usage, and heightened focus on bioengineered materials for improved patient outcomes.