Artificial Urinary Sphincter Implantation Device Market Report
Published Date: 31 January 2026 | Report Code: artificial-urinary-sphincter-implantation-device
Artificial Urinary Sphincter Implantation Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides a detailed analysis of the Artificial Urinary Sphincter Implantation Device market from 2023 to 2033, highlighting market size, growth trends, segmentation, regional insights, and key player contributions. The aim is to provide valuable forecasts and insights for stakeholders.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
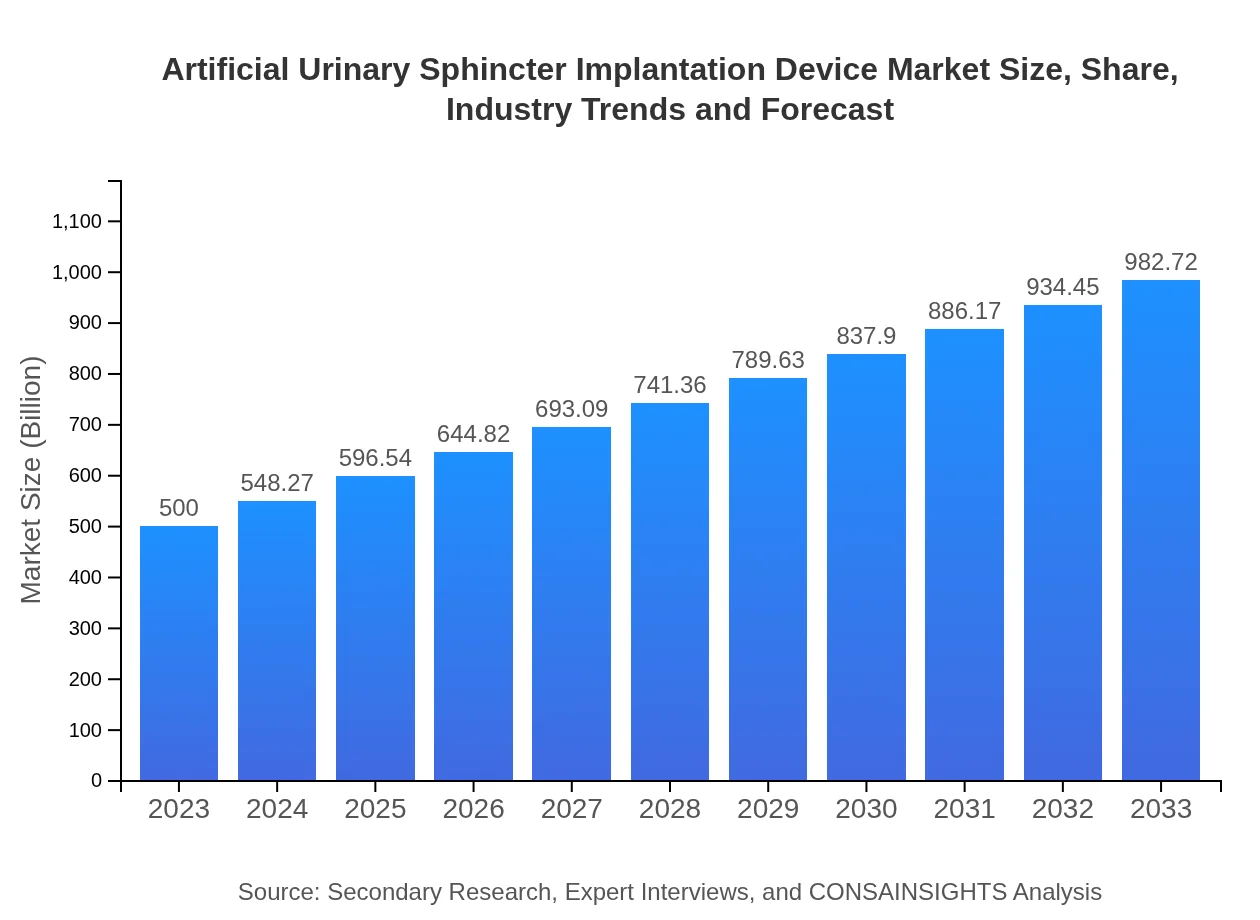
| 2023 Market Size | $500.00 Million |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $982.72 Million |
| Top Companies | Austin Systems, Shure Systems |
| Last Modified Date | 31 January 2026 |
Artificial Urinary Sphincter Implantation Device Market Overview
Customize Artificial Urinary Sphincter Implantation Device Market Report market research report
- ✔ Get in-depth analysis of Artificial Urinary Sphincter Implantation Device market size, growth, and forecasts.
- ✔ Understand Artificial Urinary Sphincter Implantation Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Artificial Urinary Sphincter Implantation Device
What is the Market Size & CAGR of Artificial Urinary Sphincter Implantation Device market in 2023-2033?
Artificial Urinary Sphincter Implantation Device Industry Analysis
Artificial Urinary Sphincter Implantation Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Artificial Urinary Sphincter Implantation Device Market Analysis Report by Region
Europe Artificial Urinary Sphincter Implantation Device Market Report:
The European market is also substantial, growing from USD 147.15 million in 2023 to USD 289.21 million by 2033. The growing aging population and increasing awareness about surgical options are crucial factors for market growth in countries like Germany, UK, and France.Asia Pacific Artificial Urinary Sphincter Implantation Device Market Report:
The Asia Pacific market is projected to grow from USD 99.10 million in 2023 to USD 194.77 million by 2033. The rise in healthcare expenditure and the increasing adoption of advanced surgical devices are key drivers in this region. Countries like India and China are seeing a higher incidence of urinary disorders, leading to increased demand.North America Artificial Urinary Sphincter Implantation Device Market Report:
North America leads the market with projected growth from USD 167.85 million in 2023 to USD 329.90 million by 2033, driven by high healthcare spending, robust R&D capabilities, and a greater focus on enhancing quality of life for patients with urinary incontinence.South America Artificial Urinary Sphincter Implantation Device Market Report:
In South America, the market is expected to increase from USD 16.65 million in 2023 to USD 32.72 million by 2033. Economic improvements and advancements in healthcare infrastructure are fostering growth, particularly in Brazil and Argentina.Middle East & Africa Artificial Urinary Sphincter Implantation Device Market Report:
For the Middle East and Africa, the market is expected to rise from USD 69.25 million in 2023 to USD 136.11 million by 2033. Factors such as increasing disposable income, advancements in medical technology, and improved healthcare services play a significant role in driving growth in this region.Tell us your focus area and get a customized research report.
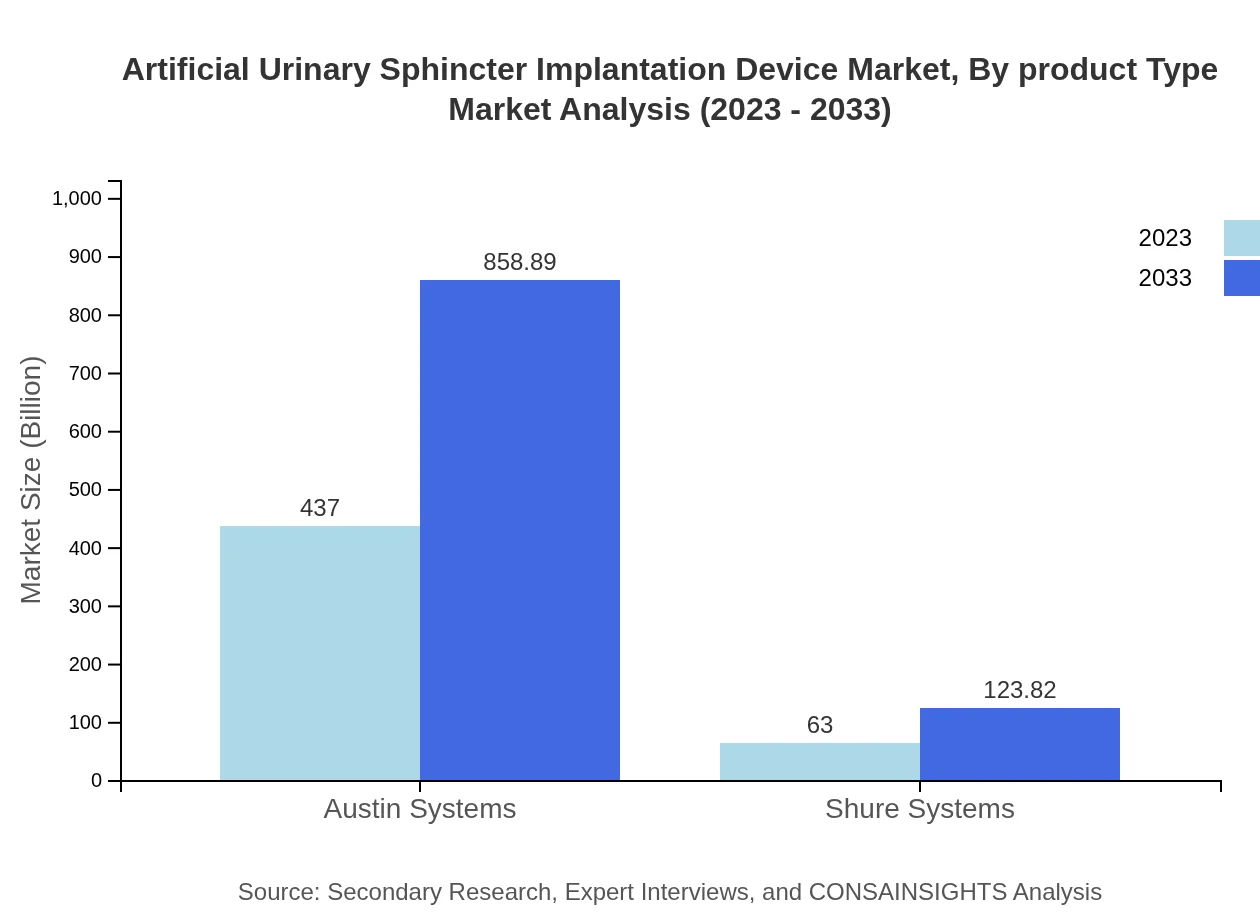
Artificial Urinary Sphincter Implantation Device Market Analysis By Product Type
The market can be categorized into mechanical and electronic devices. Mechanical devices dominated the market in 2023, accounting for 87.4% of the market share and predicted to grow significantly by 2033. Electronic devices, while smaller in size, are expected to gain traction due to their advanced functionalities and ease of use.
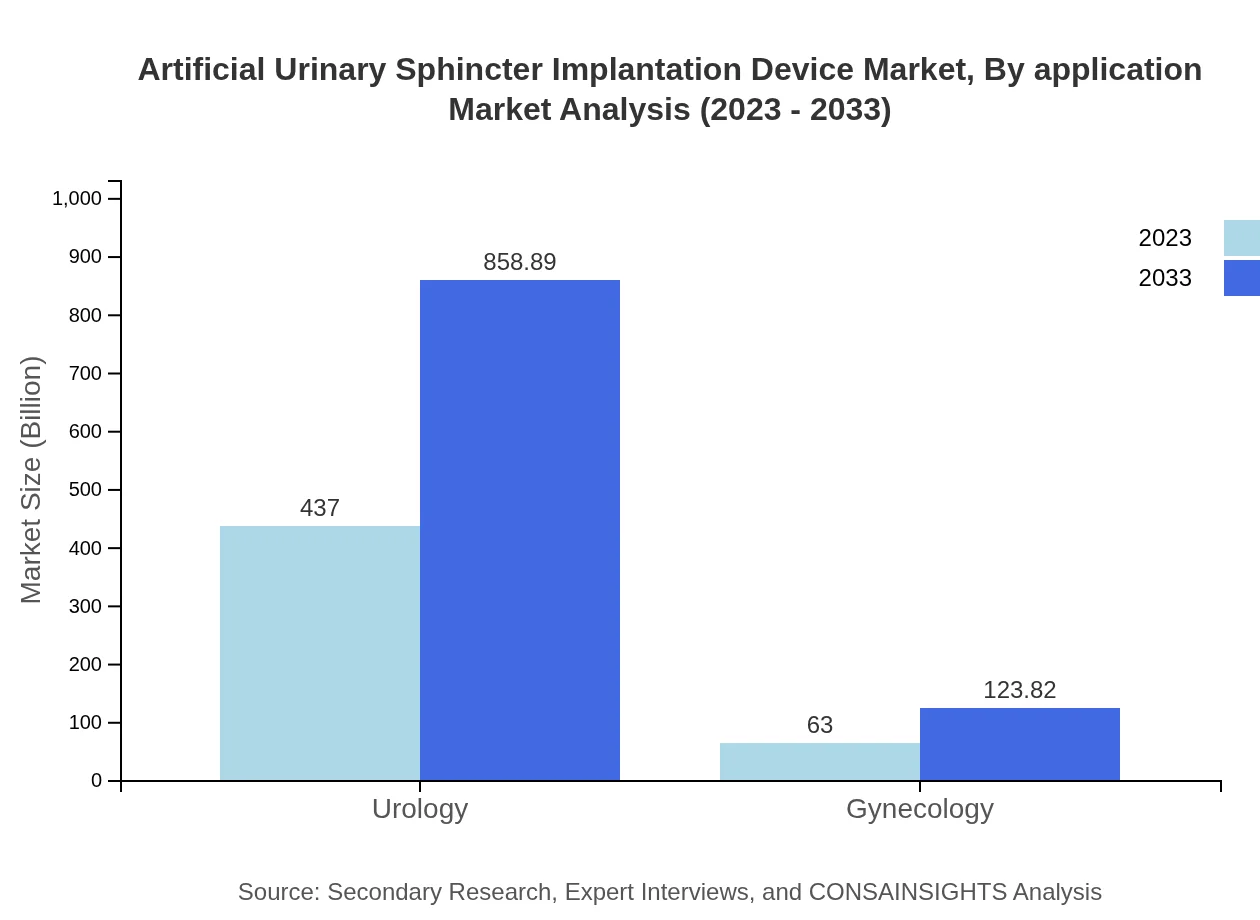
Artificial Urinary Sphincter Implantation Device Market Analysis By Application
The applications of these devices primarily include Urology and Gynecology. The Urology segment holds the largest market share, contributing 87.4% in 2023 and anticipated to continue at this rate as men suffering from urinary incontinence increasingly seek surgical solutions.
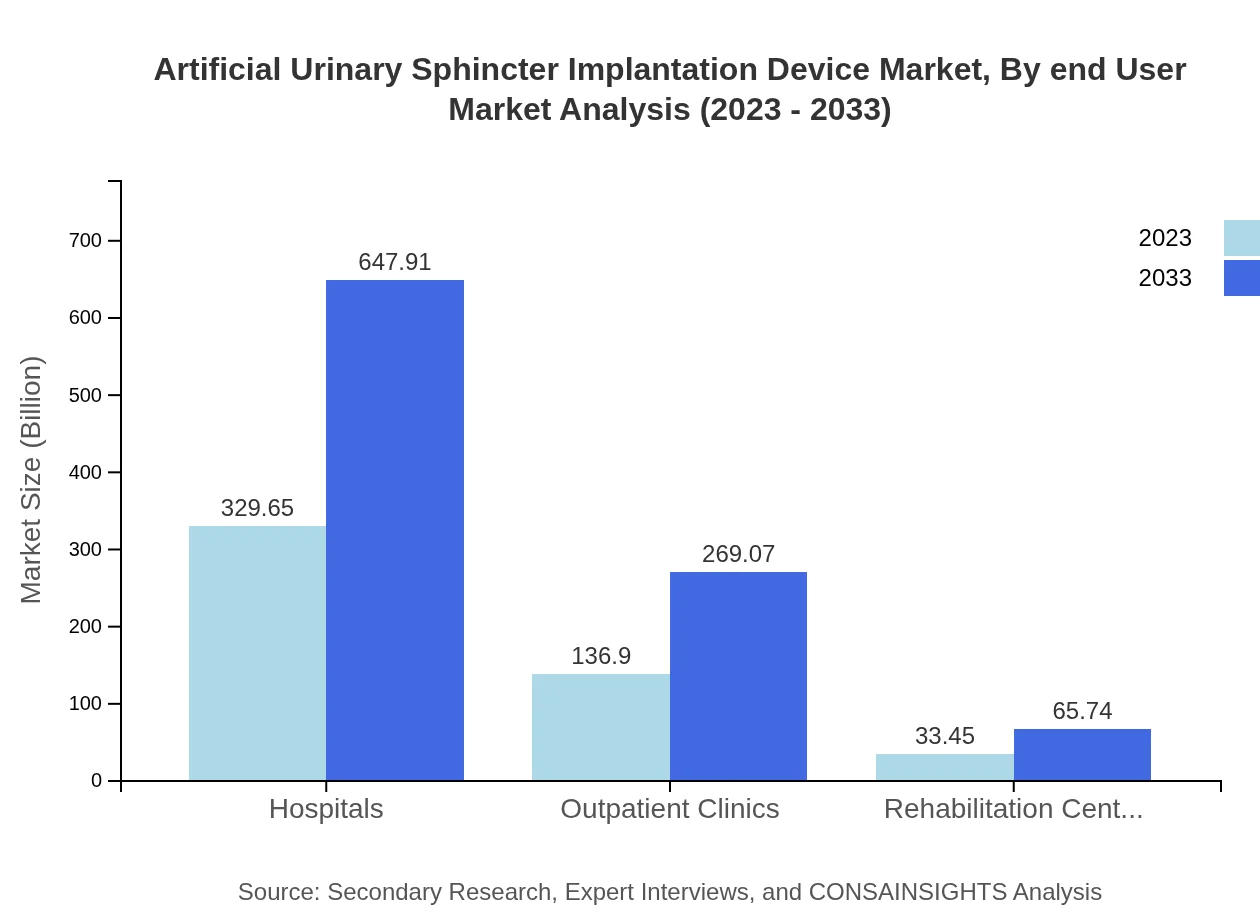
Artificial Urinary Sphincter Implantation Device Market Analysis By End User
The end-user segmentation reveals significant usage within hospitals (65.93%), outpatient clinics (27.38%), and rehabilitation centers (6.69%). Hospitals are expected to maintain their dominant market position over the next decade.
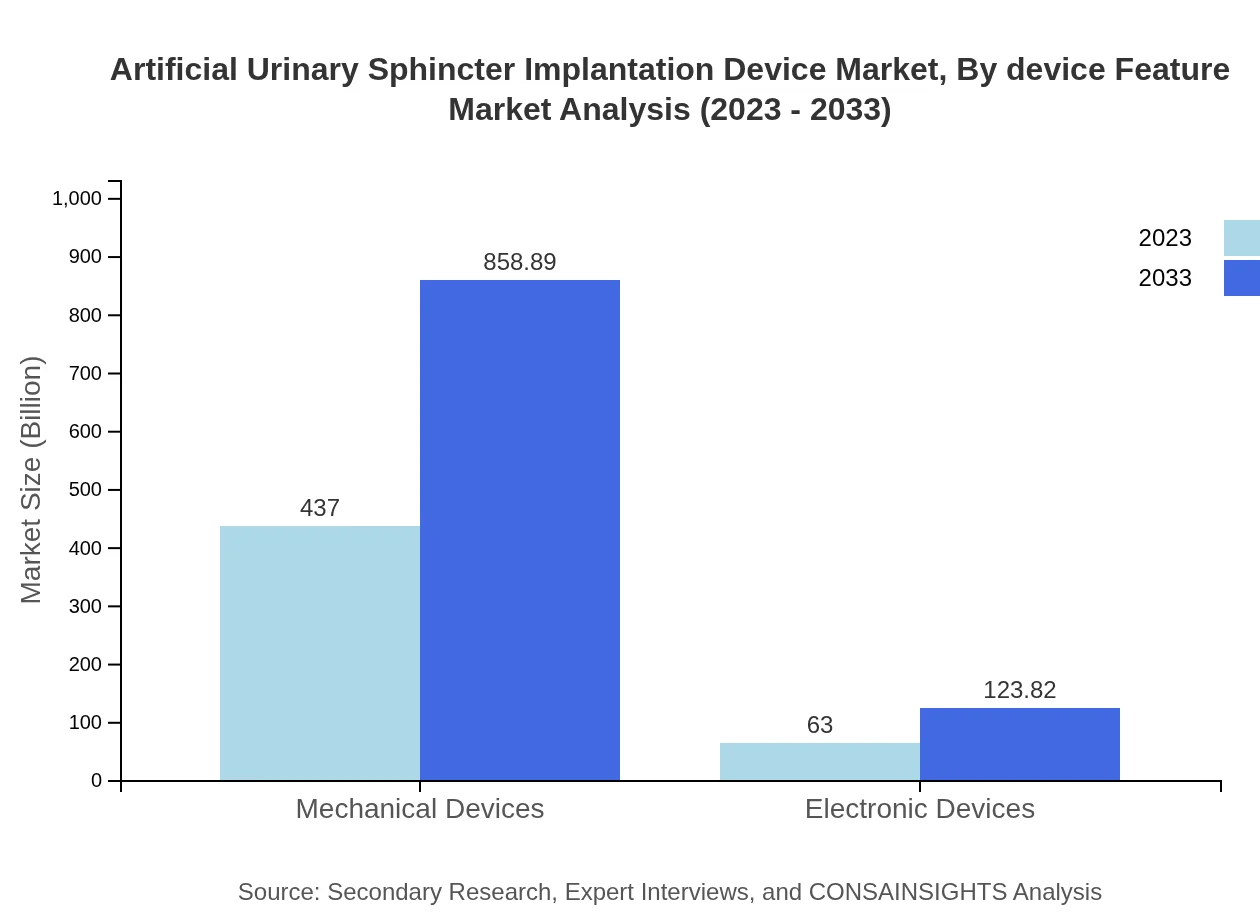
Artificial Urinary Sphincter Implantation Device Market Analysis By Device Feature
The market's device feature segmentation includes durability, adaptability, and usability. Products offering better durability and adaptability to patient-specific needs are expected to lead market growth.
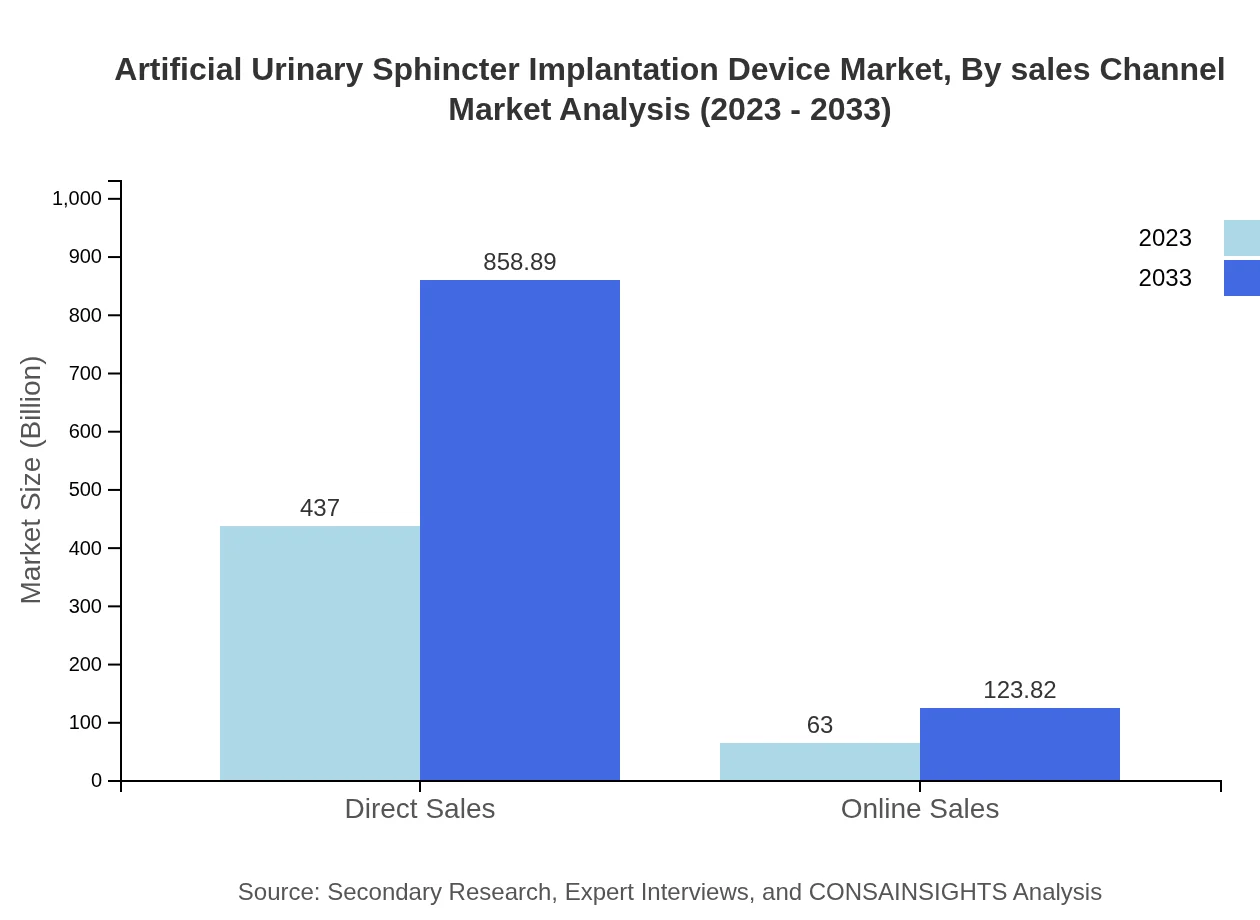
Artificial Urinary Sphincter Implantation Device Market Analysis By Sales Channel
Sales channels include direct sales and online sales. Direct sales continue to dominate due to existing hospital contracts, but online platforms are gradually gaining traction, especially among smaller clinics looking for cost-effective solutions.
Artificial Urinary Sphincter Implantation Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Artificial Urinary Sphincter Implantation Device Industry
Austin Systems:
A leading manufacturer focused on innovative solutions for urinary disorders, known for effective mechanical devices.Shure Systems:
Specializes in electronic devices that enhance patient comfort and provide significant advancements in surgical procedures.We're grateful to work with incredible clients.









FAQs
What is the market size of artificial Urinary Sphincter Implantation Device?
The global market size for Artificial Urinary Sphincter Implantation Devices is estimated at $500 million in 2023, with a projected CAGR of 6.8%, indicating a strong growth trajectory through 2033.
What are the key market players or companies in this artificial Urinary Sphincter Implantation Device industry?
Key players in the artificial urinary sphincter implantation device market include major companies specializing in urology, such as Austin Systems and Shure Systems, which lead in technology and product development.
What are the primary factors driving the growth in the artificial Urinary Sphincter Implantation Device industry?
Growth in this market is primarily driven by an increasing prevalence of urinary incontinence, advances in minimally invasive surgical techniques, and rising awareness of treatment options among healthcare providers and patients.
Which region is the fastest Growing in the artificial Urinary Sphincter Implantation Device?
The Asia Pacific region is anticipated to be the fastest-growing market for artificial urinary sphincter implantation devices, with market size expected to grow from $99.10 million in 2023 to $194.77 million in 2033.
Does ConsaInsights provide customized market report data for the artificial Urinary Sphincter Implantation Device industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the artificial urinary sphincter implantation device industry, ensuring relevant insights for strategic decision-making.
What deliverables can I expect from this artificial Urinary Sphincter Implantation Device market research project?
Deliverables from the artificial urinary sphincter implantation device market research include detailed reports, data analysis, trend insights, competitive landscape evaluations, and tailored recommendations based on market findings.
What are the market trends of artificial Urinary Sphincter Implantation Device?
Key market trends include increasing adoption of advanced surgical procedures, growth in outpatient clinics, and a shift towards more effective and patient-friendly urinary incontinence treatments.