Autoimmune And Inflammatory Immunomodulators Market Report
Published Date: 31 January 2026 | Report Code: autoimmune-and-inflammatory-immunomodulators
Autoimmune And Inflammatory Immunomodulators Market Size, Share, Industry Trends and Forecast to 2033
This market report analyzes the Autoimmune and Inflammatory Immunomodulators sector, providing insights on current trends, market size, growth forecasts, and regional performance from 2023 to 2033. It aims to equip stakeholders with comprehensive data for strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
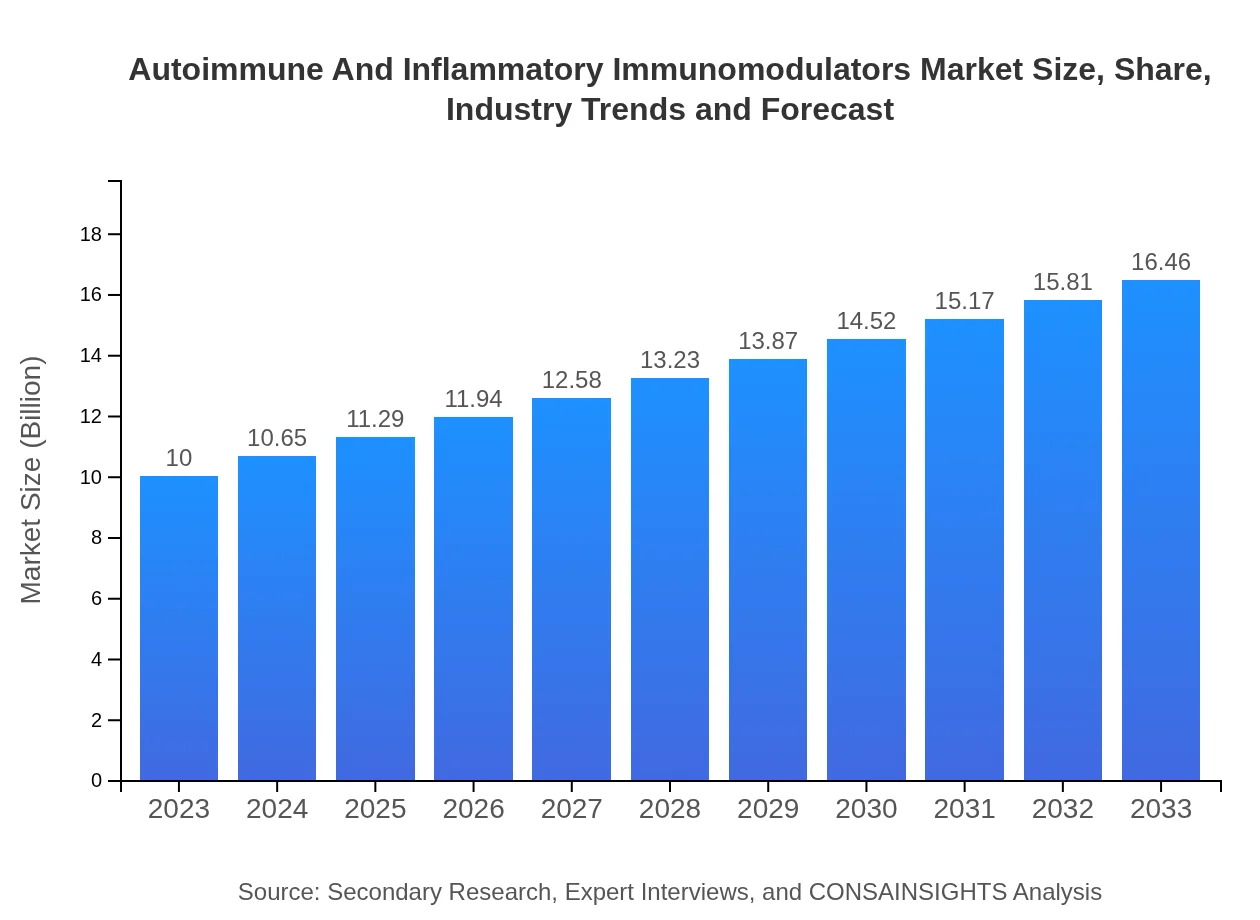
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5% |
| 2033 Market Size | $16.46 Billion |
| Top Companies | AbbVie Inc., Roche Holding AG, Johnson & Johnson, Novartis AG, Amgen Inc. |
| Last Modified Date | 31 January 2026 |
Autoimmune And Inflammatory Immunomodulators Market Overview
Customize Autoimmune And Inflammatory Immunomodulators Market Report market research report
- ✔ Get in-depth analysis of Autoimmune And Inflammatory Immunomodulators market size, growth, and forecasts.
- ✔ Understand Autoimmune And Inflammatory Immunomodulators's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Autoimmune And Inflammatory Immunomodulators
What is the Market Size & CAGR of Autoimmune And Inflammatory Immunomodulators market in 2023?
Autoimmune And Inflammatory Immunomodulators Industry Analysis
Autoimmune And Inflammatory Immunomodulators Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Autoimmune And Inflammatory Immunomodulators Market Analysis Report by Region
Europe Autoimmune And Inflammatory Immunomodulators Market Report:
In Europe, the market for Autoimmune and Inflammatory Immunomodulators is expected to grow from USD 2.62 billion in 2023 to USD 4.31 billion by 2033. Factors such as favorable regulatory environments and a high concentration of key market players enable growth, alongside increasing prevalence of chronic diseases.Asia Pacific Autoimmune And Inflammatory Immunomodulators Market Report:
The Asia Pacific region is anticipated to experience robust growth in the autoimmunity market, fueled by increasing healthcare investments and a rising population suffering from autoimmune disorders. The market size is expected to grow from USD 1.94 billion in 2023 to USD 3.19 billion by 2033, reflecting a CAGR of 5.05%. Nations like China and India are driving this growth through expanding healthcare infrastructure and rising disposable incomes.North America Autoimmune And Inflammatory Immunomodulators Market Report:
North America is the largest market for Autoimmune and Inflammatory Immunomodulators, with a market size set to rise from USD 3.80 billion in 2023 to USD 6.25 billion in 2033, reflecting a CAGR of 5.09%. Advanced healthcare systems, high prevalence of autoimmune diseases, and extensive R&D investments by pharmaceutical giants contribute to this dominance.South America Autoimmune And Inflammatory Immunomodulators Market Report:
In South America, the autoimmune and inflammatory immunomodulators market shows potential for growth despite economic challenges, with market size projected to expand from USD 0.31 billion in 2023 to USD 0.52 billion by 2033. This growth is supported by increasing awareness of autoimmune diseases and enhanced access to healthcare services in countries like Brazil and Argentina.Middle East & Africa Autoimmune And Inflammatory Immunomodulators Market Report:
The Middle East and Africa are expecting growth in the Autoimmune and Inflammatory Immunomodulators market, increasing from USD 1.33 billion in 2023 to USD 2.19 billion by 2033. This growth is driven by improved healthcare services and greater awareness of autoimmune diseases amid a rapidly growing population.Tell us your focus area and get a customized research report.
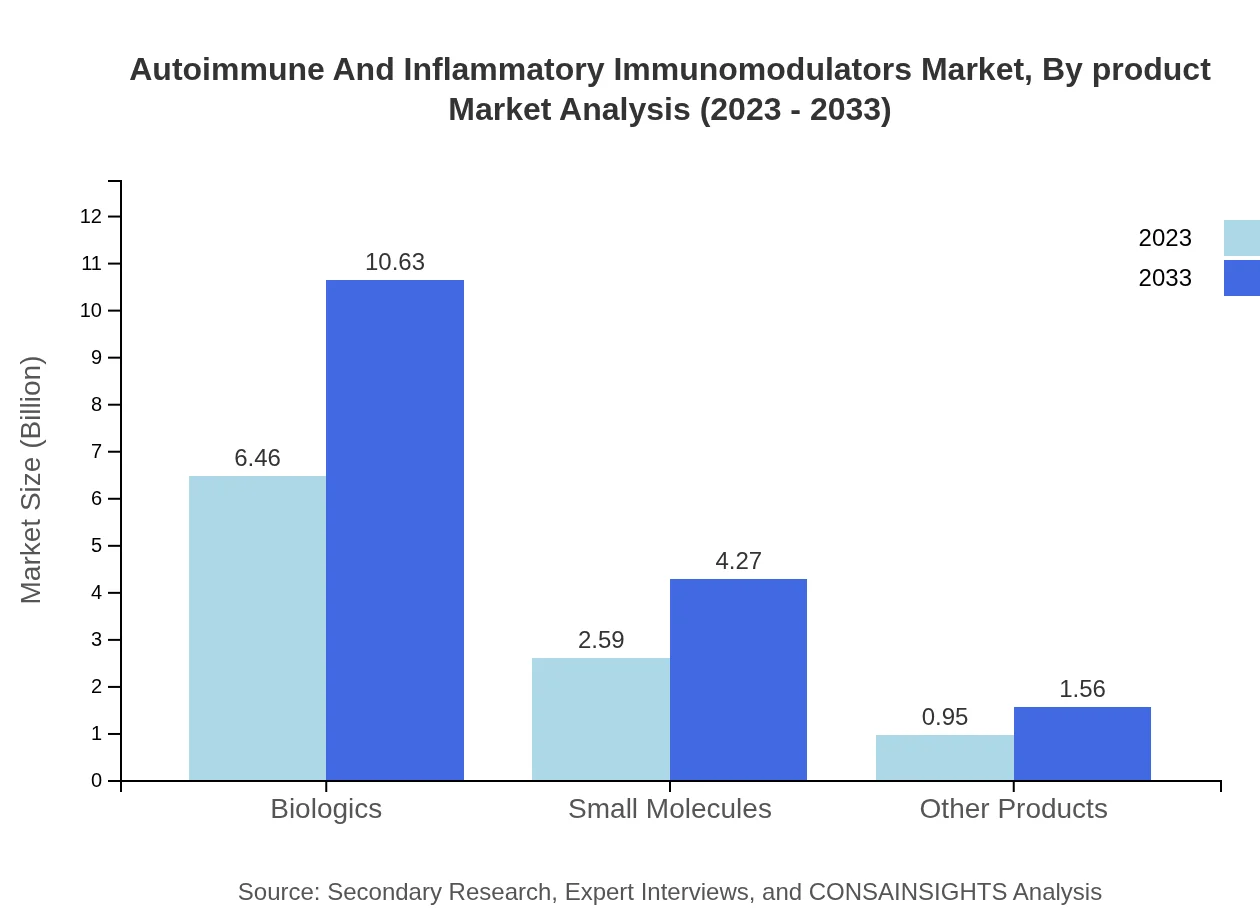
Autoimmune And Inflammatory Immunomodulators Market Analysis By Product
The global market for Autoimmune and Inflammatory Immunomodulators by product type is dominated by biologics, expected to grow from USD 6.46 billion in 2023 to USD 10.63 billion by 2033. Small molecules also hold significant market share, with an increase from USD 2.59 billion to USD 4.27 billion. Other products, while smaller in share, are expected to grow from USD 0.95 billion to USD 1.56 billion during the forecast period.
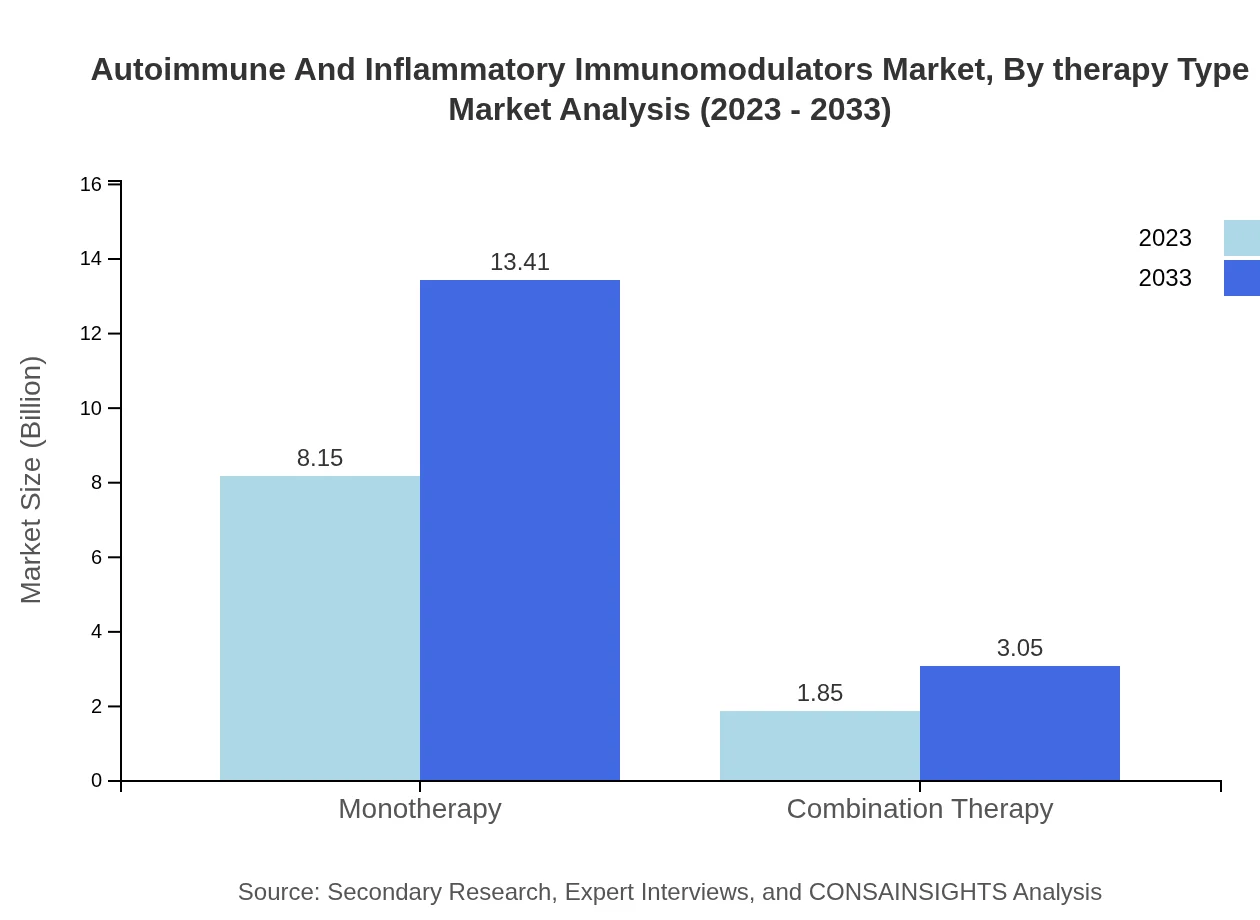
Autoimmune And Inflammatory Immunomodulators Market Analysis By Therapy Type
Monotherapy represents the largest share of the market, with projections for growth from USD 8.15 billion in 2023 to USD 13.41 billion by 2033. Meanwhile, combination therapy is anticipated to grow steadily from USD 1.85 billion to USD 3.05 billion, catering to the evolving treatment landscape.
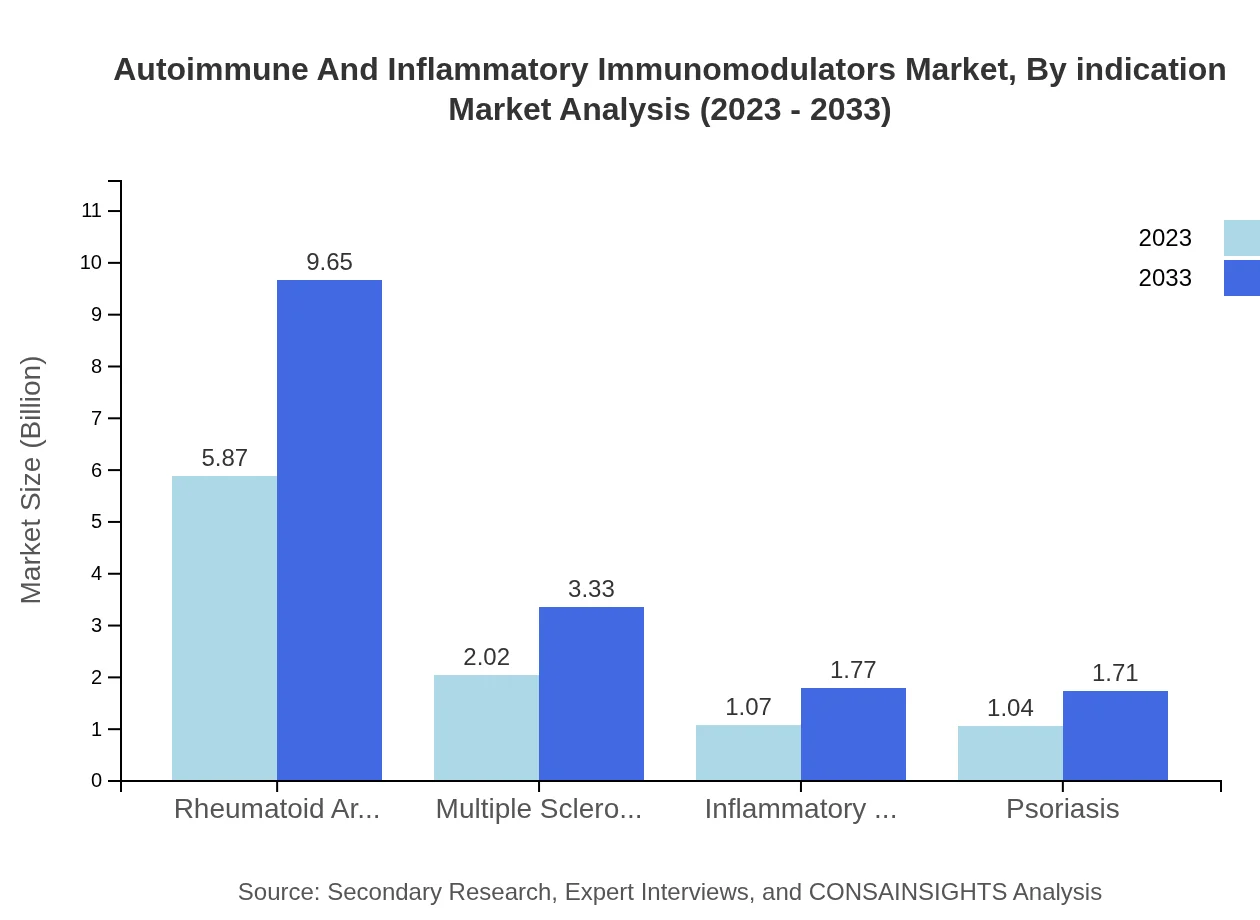
Autoimmune And Inflammatory Immunomodulators Market Analysis By Indication
The market is significantly influenced by indications such as rheumatoid arthritis, projected to grow from USD 5.87 billion in 2023 to USD 9.65 billion by 2033. Additional segments like multiple sclerosis and inflammatory bowel disease also show promise, although at a smaller scale.
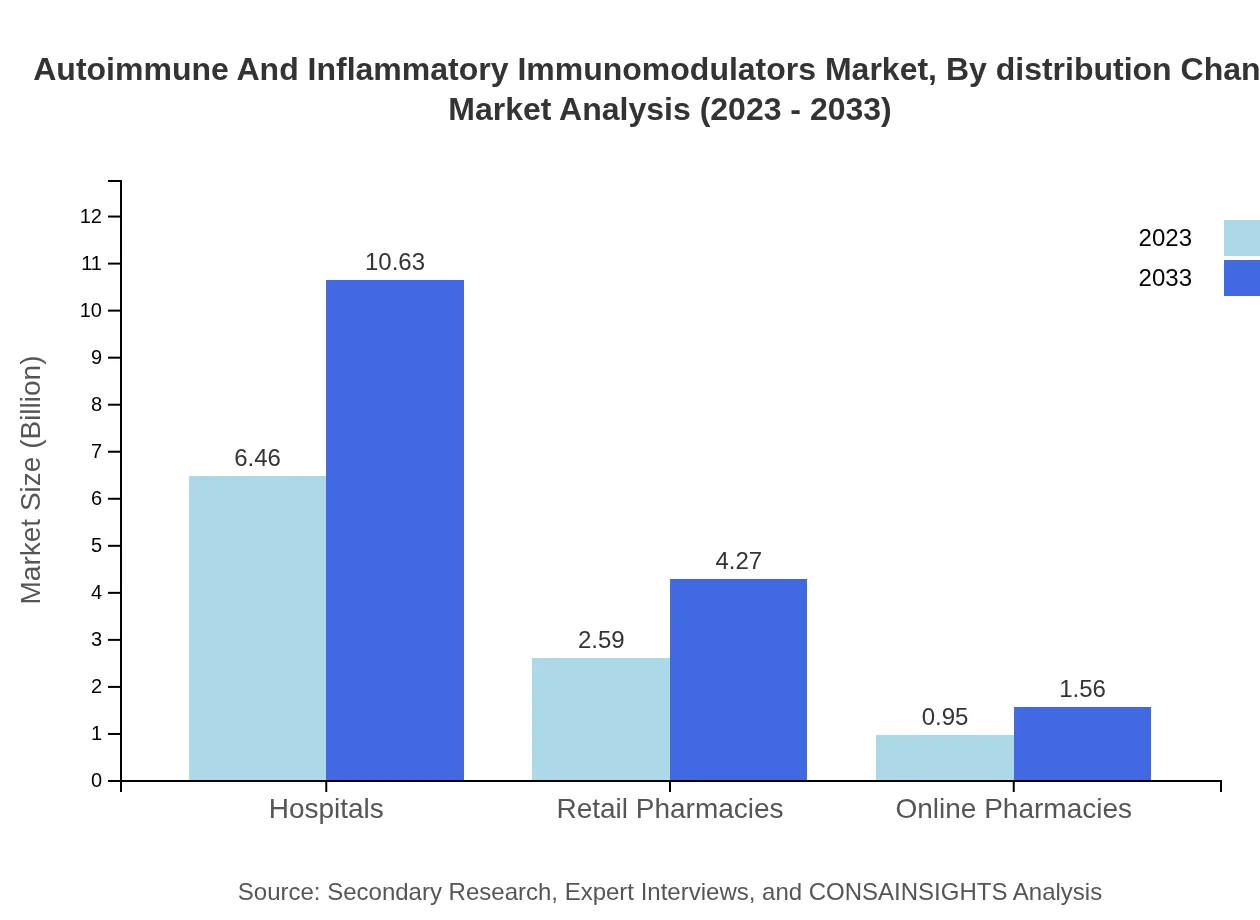
Autoimmune And Inflammatory Immunomodulators Market Analysis By Distribution Channel
Distribution channels across the market include hospitals, retail pharmacies, and online pharmacies. Hospitals lead this segment with sizes increasing from USD 6.46 billion to USD 10.63 billion, while retail pharmacies and online channels also expand their footprints in response to changing consumer preferences.
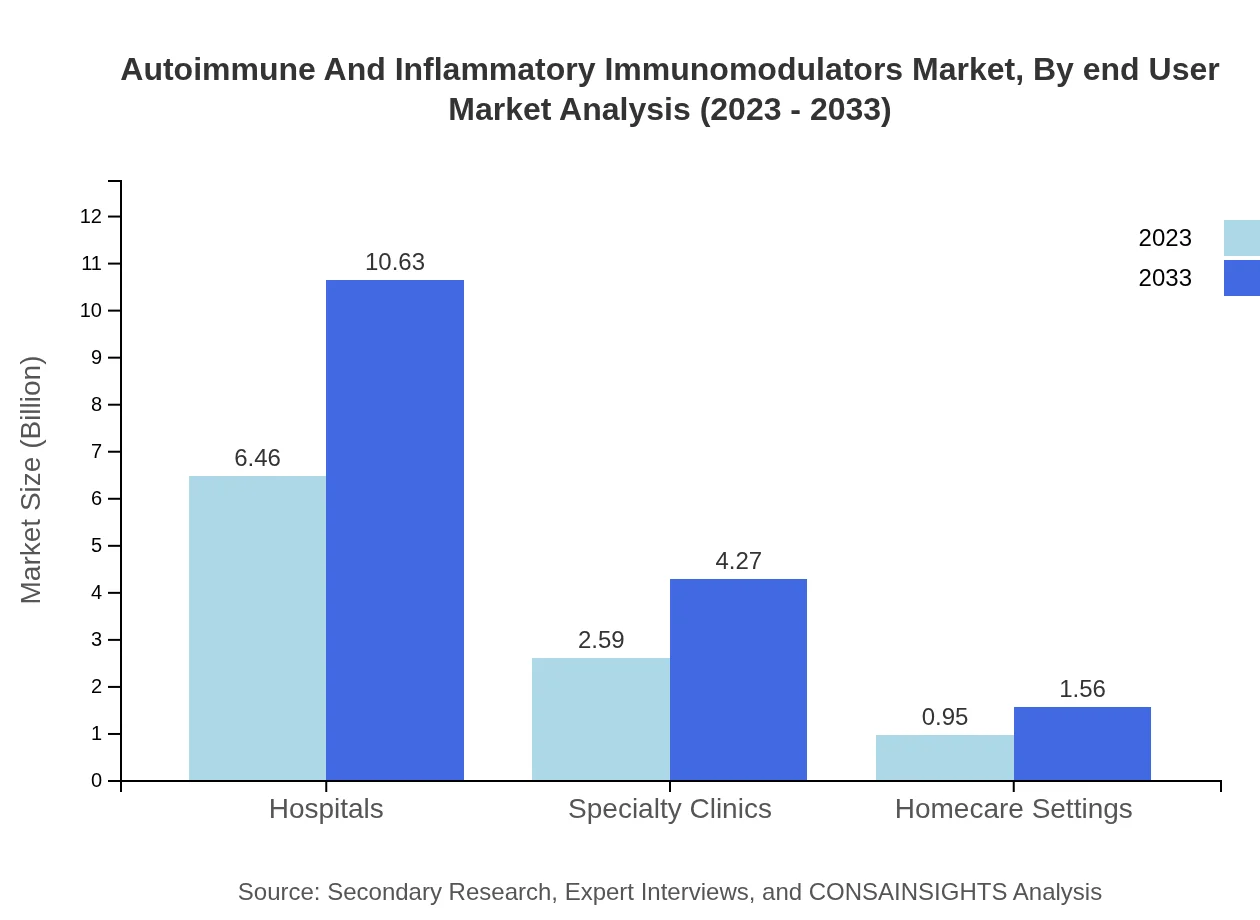
Autoimmune And Inflammatory Immunomodulators Market Analysis By End User
The end-user segment predominantly consists of hospitals, which represent a significant share of the market. The increase in homecare settings offers additional avenues for market growth, as patients prefer at-home management of their conditions, particularly amid evolving healthcare dynamics.
Autoimmune And Inflammatory Immunomodulators Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Autoimmune And Inflammatory Immunomodulators Industry
AbbVie Inc.:
AbbVie is a leading global biopharmaceutical company known for its innovative treatments in autoimmune diseases, especially Humira, which has a strong market presence.Roche Holding AG:
Roche's products, such as Ocrevus and Actemra, are critical in managing autoimmune conditions, showcasing the company's deep investment in research and development.Johnson & Johnson:
Johnson & Johnson offers a diversified portfolio with products like Remicade, playing a significant role in the autoimmune therapeutics landscape.Novartis AG:
With its strong focus on innovation, Novartis has developed key treatments for autoimmune and inflammatory diseases, including Cosentyx.Amgen Inc.:
Amgen is recognized for its biosimilar and innovative therapies in the autoimmune space, contributing to the accessibility of treatments for patients globally.We're grateful to work with incredible clients.









FAQs
What is the market size of autoimmune and inflammatory immunomodulators?
The autoimmune and inflammatory immunomodulators market is projected to reach $10 billion by 2033, with a CAGR of 5%. This growth indicates the increasing demand for therapies targeting autoimmune diseases and inflammatory conditions.
What are the key market players or companies in this industry?
Key players in the autoimmune and inflammatory immunomodulators market include leading pharmaceutical companies such as AbbVie, Amgen, Merck, and Novartis, which are actively involved in developing innovative therapies and biologics.
What are the primary factors driving the growth in the autoimmune and inflammatory immunomodulators industry?
Growth in the autoimmune and inflammatory immunomodulators market is driven by the rising incidence of autoimmune disorders, advancements in biologic therapies, and increasing R&D investments by pharmaceutical companies to develop novel treatments.
Which region is the fastest Growing in the autoimmune and inflammatory immunomodulators market?
The fastest-growing region in the autoimmune and inflammatory immunomodulators market is North America. The market is expected to grow from $3.80 billion in 2023 to $6.25 billion by 2033, reflecting a significant increase.
Does ConsaInsights provide customized market report data for the autoimmune and inflammatory immunomodulators industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs within the autoimmune and inflammatory immunomodulators industry, allowing clients to gain in-depth insights and analysis.
What deliverables can I expect from this market research project?
Clients can expect comprehensive market analysis reports, segmentation data, growth forecasts, competitive landscape assessments, and region-specific insights to make informed business decisions in the autoimmune and inflammatory immunomodulators market.
What are the market trends of autoimmune and inflammatory immunomodulators?
Current trends in the autoimmune and inflammatory immunomodulators market include an increasing shift towards biologics, a rise in demand for personalized medicine, and innovative drug delivery systems to enhance patient outcomes.