Biliary Biopsy Forceps Market Report
Published Date: 31 January 2026 | Report Code: biliary-biopsy-forceps
Biliary Biopsy Forceps Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Biliary Biopsy Forceps market, exploring trends, key segments, technological advancements, and a forecast for the market from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
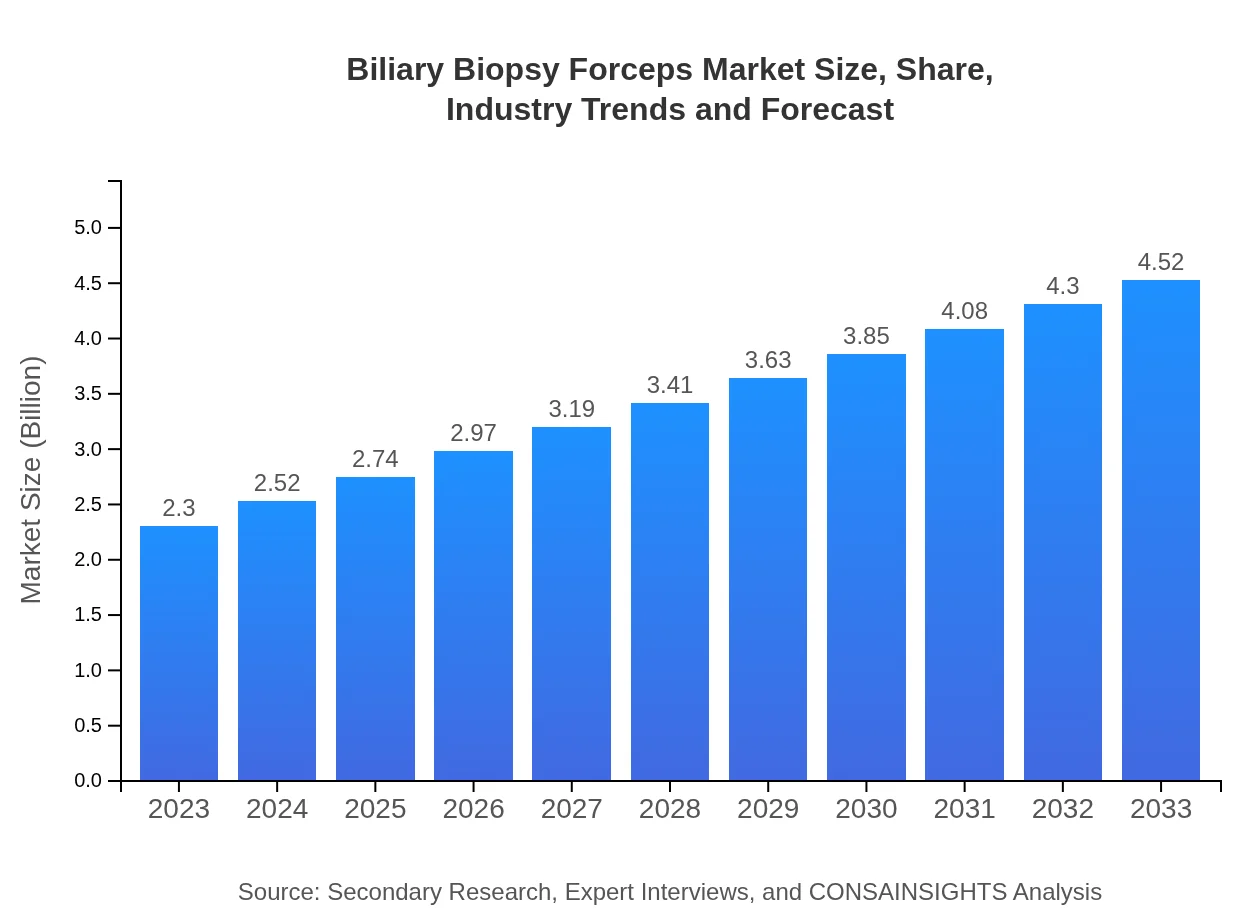
| 2023 Market Size | $2.30 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.52 Billion |
| Top Companies | Medtronic , Boston Scientific, Olympus Corporation, Cook Medical |
| Last Modified Date | 31 January 2026 |
Biliary Biopsy Forceps Market Overview
Customize Biliary Biopsy Forceps Market Report market research report
- ✔ Get in-depth analysis of Biliary Biopsy Forceps market size, growth, and forecasts.
- ✔ Understand Biliary Biopsy Forceps's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biliary Biopsy Forceps
What is the Market Size & CAGR of Biliary Biopsy Forceps market in 2023?
Biliary Biopsy Forceps Industry Analysis
Biliary Biopsy Forceps Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biliary Biopsy Forceps Market Analysis Report by Region
Europe Biliary Biopsy Forceps Market Report:
The European market for Biliary Biopsy Forceps is poised to grow from $0.76 billion in 2023 to $1.49 billion by 2033, with a CAGR of 7%. The increase is driven by aging populations, increasing surgical interventions, and a rising number of gastroenterology practitioners offering advanced therapies.Asia Pacific Biliary Biopsy Forceps Market Report:
In the Asia-Pacific region, the Biliary Biopsy Forceps market is expected to grow from $0.44 billion in 2023 to $0.86 billion by 2033, reflecting a steady CAGR of 7.1%. The growth is driven by increasing healthcare infrastructure investments, rising disposable incomes, and a growing population which adds to the prevalence of biliary diseases.North America Biliary Biopsy Forceps Market Report:
North America dominates the Biliary Biopsy Forceps market, with a size of $0.75 billion in 2023, anticipated to reach $1.47 billion by 2033. This robust growth, at a CAGR of 7.2%, is attributed to advanced healthcare facilities, high prevalence of biliary disorders, and strong focus on innovation in medical device technology.South America Biliary Biopsy Forceps Market Report:
The South American Biliary Biopsy Forceps market is projected to grow from $0.13 billion in 2023 to $0.26 billion in 2033. Factors such as improved healthcare access and rising awareness regarding biliary health are expected to drive market expansion at a CAGR of 7.5%.Middle East & Africa Biliary Biopsy Forceps Market Report:
In the Middle East and Africa, the market is set to grow from $0.22 billion in 2023 to $0.43 billion by 2033, achieving a CAGR of 7.2%. The growth is supported by government initiatives to improve healthcare infrastructure and increasing investments in medical equipment.Tell us your focus area and get a customized research report.
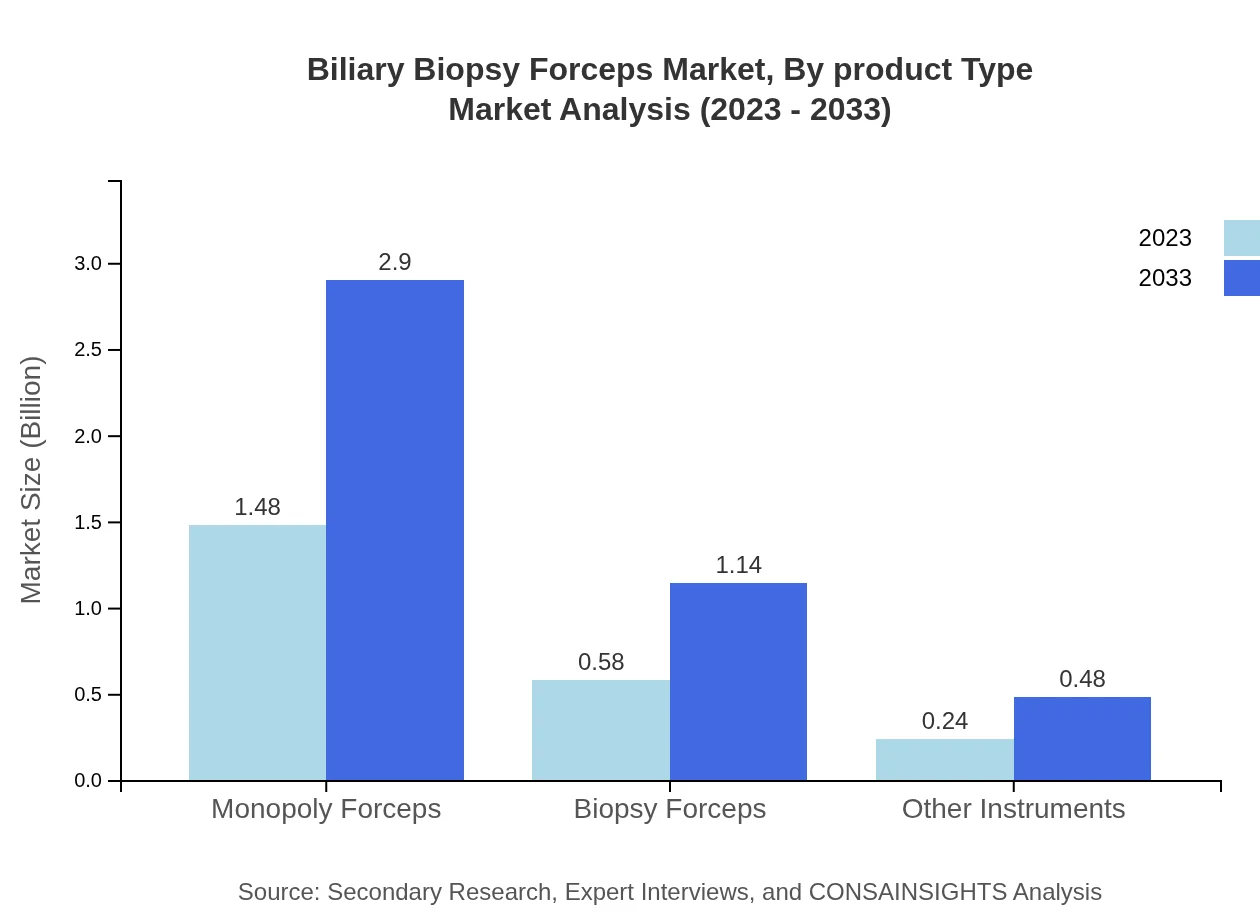
Biliary Biopsy Forceps Market Analysis By Product Type
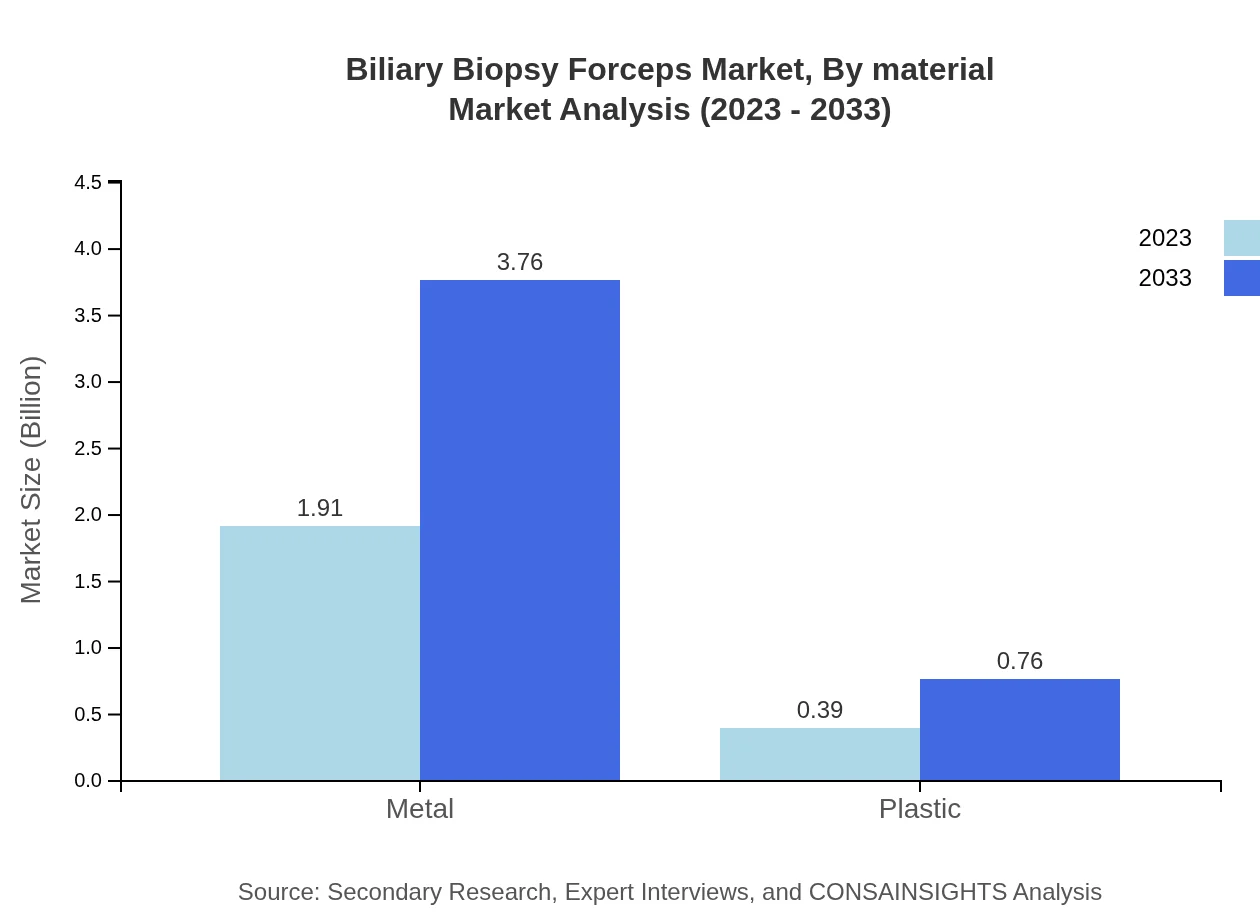
The market is primarily divided into metal and plastic Biliary Biopsy Forceps. Metal forceps dominate with a market value of $1.91 billion in 2023 and are expected to reach $3.76 billion in 2033, capturing approximately 83.08% of the market share. Plastic variants, while growing in demand, hold a smaller segment with a size of $0.39 billion in 2023, anticipated to grow to $0.76 billion by 2033, representing a market share of 16.92%.
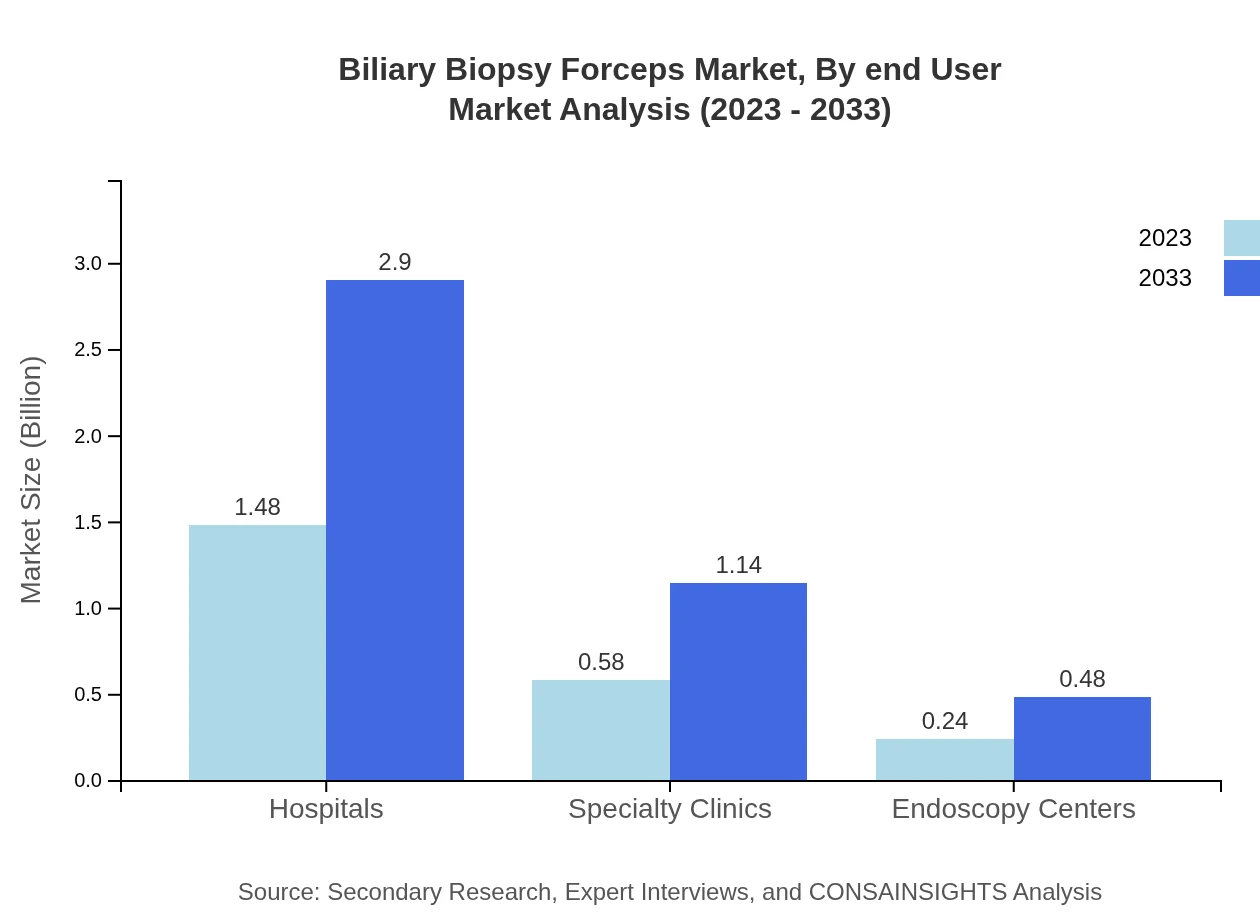
Biliary Biopsy Forceps Market Analysis By End User
The hospital setting leads the market with a projected value of $1.48 billion in 2023, expected to rise to $2.90 billion by 2033, maintaining a significant share of 64.21%. Specialty clinics and endoscopy centers are also shaping the market dynamic, projected to reach $1.14 billion in specialty clinics and $0.48 billion in endoscopy centers over the forecast period.
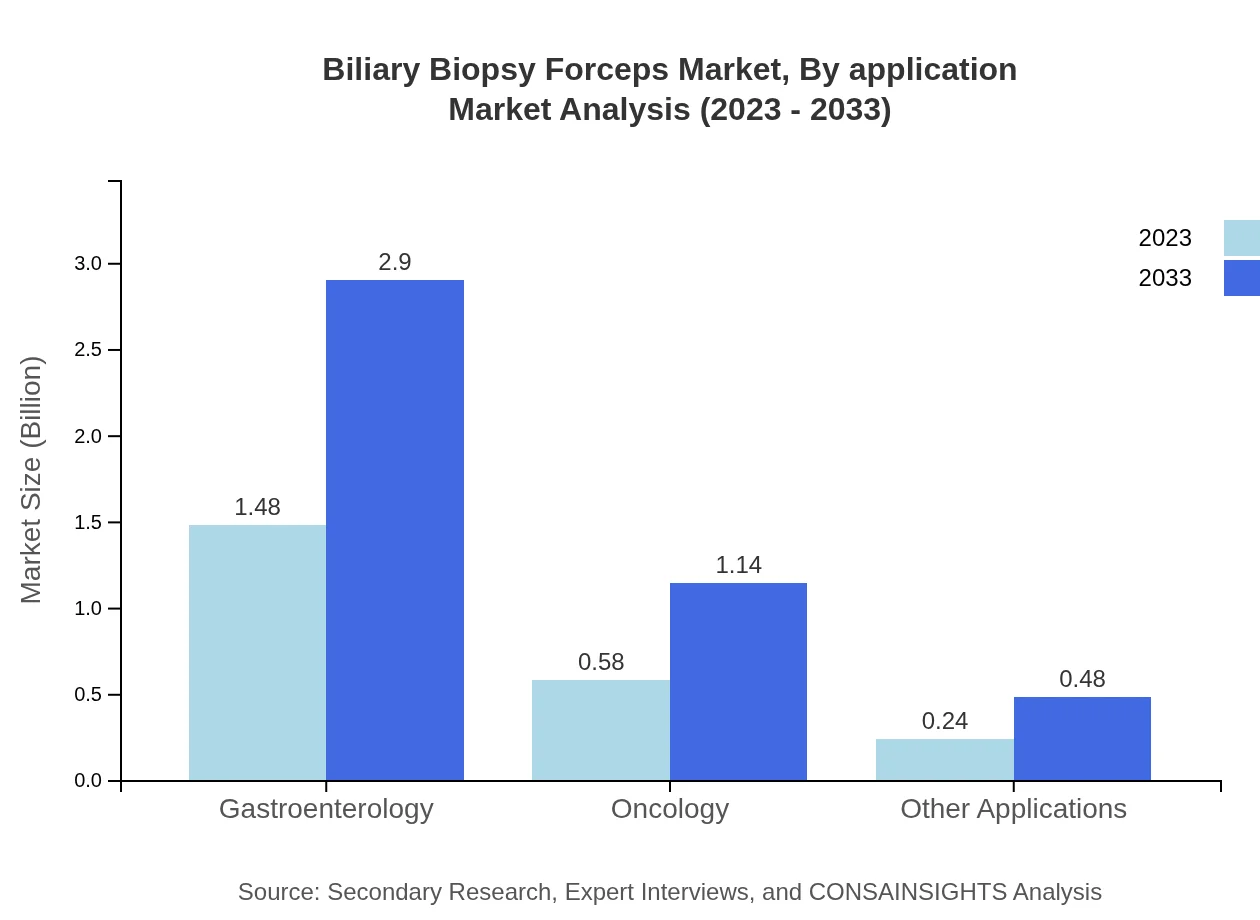
Biliary Biopsy Forceps Market Analysis By Application
Gastroenterology is the leading application area for Biliary Biopsy Forceps, valued at $1.48 billion in 2023 and anticipated to double to $2.90 billion by 2033. Oncology follows with a size of $0.58 billion, growing to $1.14 billion. Other applications also contribute, projected to reach $0.48 billion by 2033.
Biliary Biopsy Forceps Market Analysis By Material
The market is segmented into metal and plastic materials, with metal forceps expected to dominate due to their reliability and performance, growing from $1.91 billion in 2023 to $3.76 billion by 2033. Plastic forceps, though smaller in size, present an opportunity in specific applications and are expected to reach $0.76 billion.
Biliary Biopsy Forceps Market Analysis By Distribution Channel
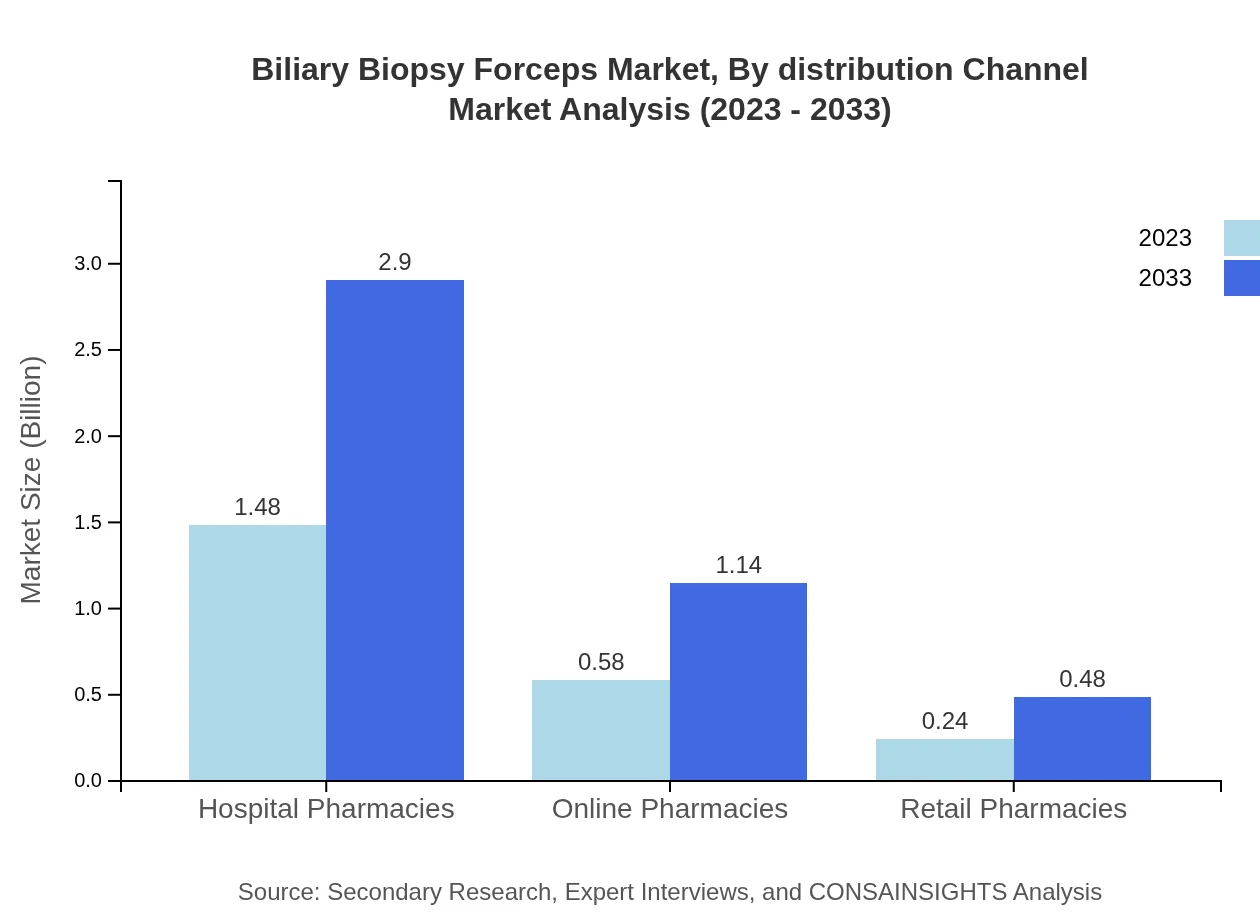
Hospital pharmacies are expected to continue dominating the distribution channels with a size of $1.48 billion in 2023, projected to rise to $2.90 billion. Online pharmacies and retail pharmacies collectively enhance market accessibility, with expected values of $1.14 billion and $0.48 billion respectively by 2033.
Biliary Biopsy Forceps Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biliary Biopsy Forceps Industry
Medtronic :
A leader in medical technology, Medtronic develops and provides innovative devices and therapies, including advanced biliary biopsy forceps that enhance surgical outcomes.Boston Scientific:
Boston Scientific is known for its pioneering medical solutions; its biopsy forceps are widely used throughout the biliary procedure spectrum, focusing on patient safety and procedural efficacy.Olympus Corporation:
A leading manufacturer of optical and digital precision technology, Olympus offers high-quality biliary biopsy forceps as part of its expansive gastrointestinal product line.Cook Medical:
Cook Medical develops specialized medical devices, including biliary biopsy forceps, contributing to minimally invasive surgical techniques that improve patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of biliary Biopsy Forceps?
The global biliary biopsy forceps market is valued at approximately $2.3 billion in 2023, with a projected Compound Annual Growth Rate (CAGR) of 6.8% through 2033, indicating significant growth in this medical device sector.
What are the key market players or companies in the biliary Biopsy Forceps industry?
Key players in the biliary biopsy forceps market include multiple leading medical device companies known for their innovative products, robust distribution networks, and strong market presence across different geographic regions.
What are the primary factors driving the growth in the biliary Biopsy Forceps industry?
Growth in the biliary biopsy forceps market is primarily driven by advancements in endoscopic technologies, increasing prevalence of biliary diseases, and a rising demand for minimally invasive procedures among healthcare professionals.
Which region is the fastest Growing in the biliary Biopsy Forceps market?
The Asia Pacific region is the fastest-growing market for biliary biopsy forceps, projected to grow from $0.44 billion in 2023 to $0.86 billion by 2033, showcasing a significant increase in healthcare investments and population health management.
Does ConsaInsights provide customized market report data for the biliary Biopsy Forceps industry?
Yes, ConsaInsights offers customized market report data tailored to specific requirements in the biliary biopsy forceps industry, allowing clients to focus on particular segments or regions for in-depth insights.
What deliverables can I expect from this biliary Biopsy Forceps market research project?
Clients can expect comprehensive deliverables including detailed market analysis, growth forecasts, competitive landscape reports, and segmentation insights to support strategic decision-making in the biliary biopsy forceps market.
What are the market trends of biliary Biopsy Forceps?
Current market trends for biliary biopsy forceps include a shift towards innovative materials, a focus on patient safety, increased use of robotic assistance in procedures, and a growing emphasis on training for medical professionals.