Biocompatibility Testing Services Market Report
Published Date: 31 January 2026 | Report Code: biocompatibility-testing-services
Biocompatibility Testing Services Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Biocompatibility Testing Services market, covering market size, growth forecasts, regional insights, segmentation analysis, and trends from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
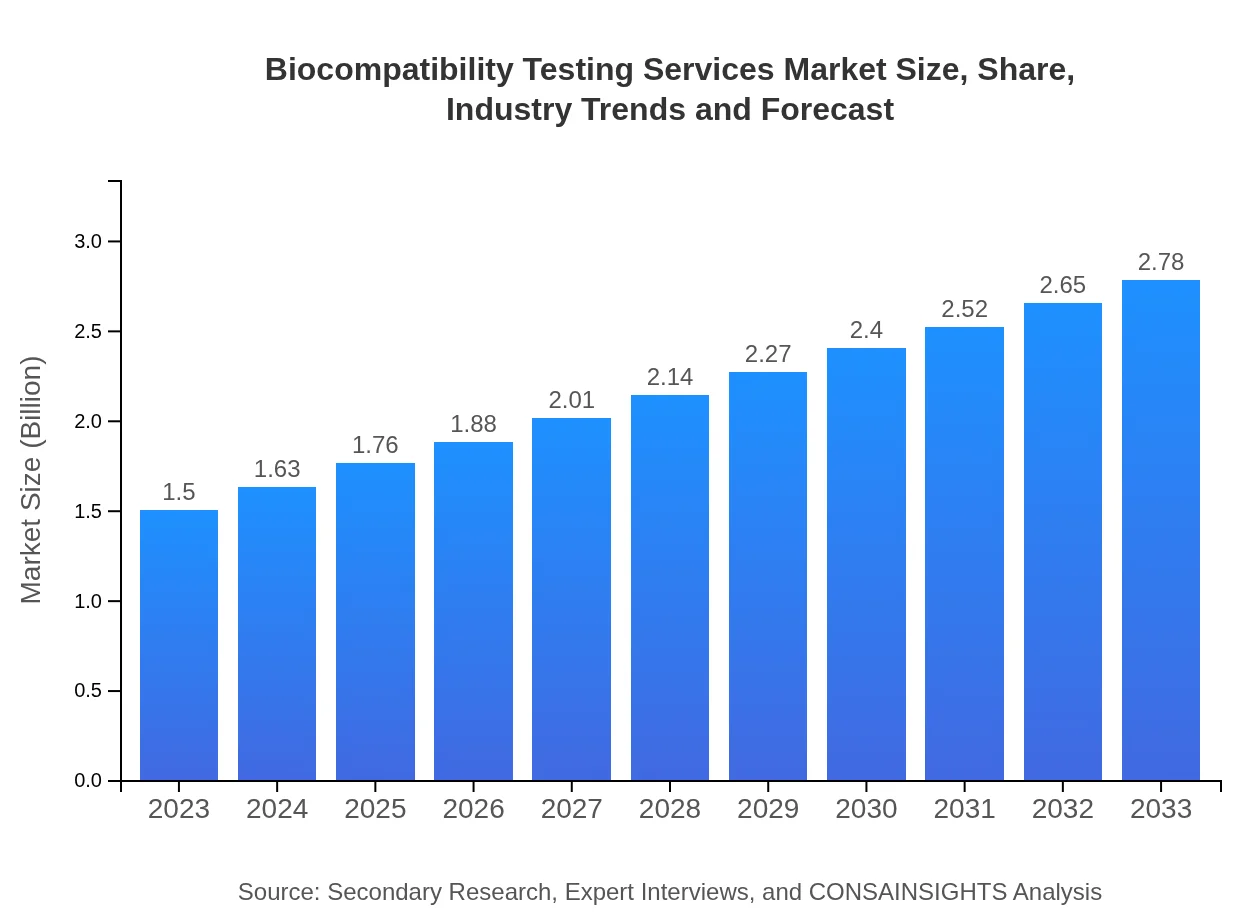
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $2.78 Billion |
| Top Companies | Eurofins Scientific, Charles River Laboratories, NAMSA, TÜV Rheinland |
| Last Modified Date | 31 January 2026 |
Biocompatibility Testing Services Market Overview
Customize Biocompatibility Testing Services Market Report market research report
- ✔ Get in-depth analysis of Biocompatibility Testing Services market size, growth, and forecasts.
- ✔ Understand Biocompatibility Testing Services's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biocompatibility Testing Services
What is the Market Size & CAGR of Biocompatibility Testing Services market in 2023?
Biocompatibility Testing Services Industry Analysis
Biocompatibility Testing Services Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biocompatibility Testing Services Market Analysis Report by Region
Europe Biocompatibility Testing Services Market Report:
Europe's market is growing steadily, projected to expand from $0.46 billion in 2023 to $0.86 billion by 2033. The presence of stringent regulatory bodies and a focus on product safety and efficacy are major drivers in this region.Asia Pacific Biocompatibility Testing Services Market Report:
The Asia Pacific region is forecasted to see substantial growth, with the market size projected to rise from $0.28 billion in 2023 to $0.51 billion by 2033. This growth is driven by the increasing investments in healthcare infrastructure and the rise in demand for advanced medical devices and materials.North America Biocompatibility Testing Services Market Report:
North America leads the market, with an increase from $0.54 billion in 2023 to $1.00 billion by 2033. The region is characterized by high healthcare expenditure, numerous medical device manufacturers, and stringent regulatory frameworks.South America Biocompatibility Testing Services Market Report:
In South America, the Biocompatibility Testing Services market is expected to grow from $0.13 billion in 2023 to $0.24 billion by 2033. The growth is attributed to the rising need for medical innovations and favorable government support towards healthcare improvements.Middle East & Africa Biocompatibility Testing Services Market Report:
In the Middle East and Africa, the market is expected to see growth from $0.09 billion in 2023 to $0.16 billion by 2033. Factors contributing to this growth include increased healthcare investments and growing awareness about biocompatibility testing.Tell us your focus area and get a customized research report.
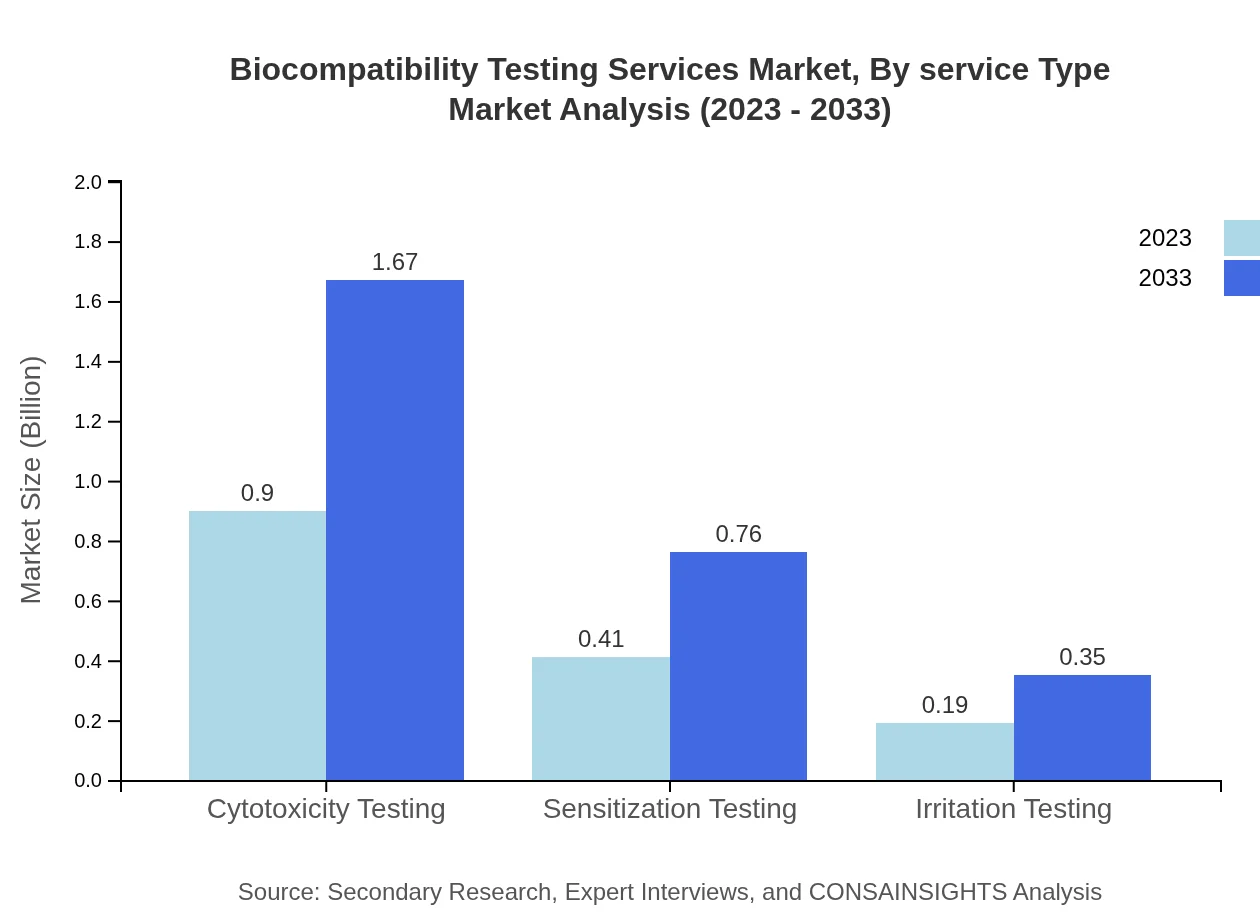
Biocompatibility Testing Services Market Analysis By Service Type
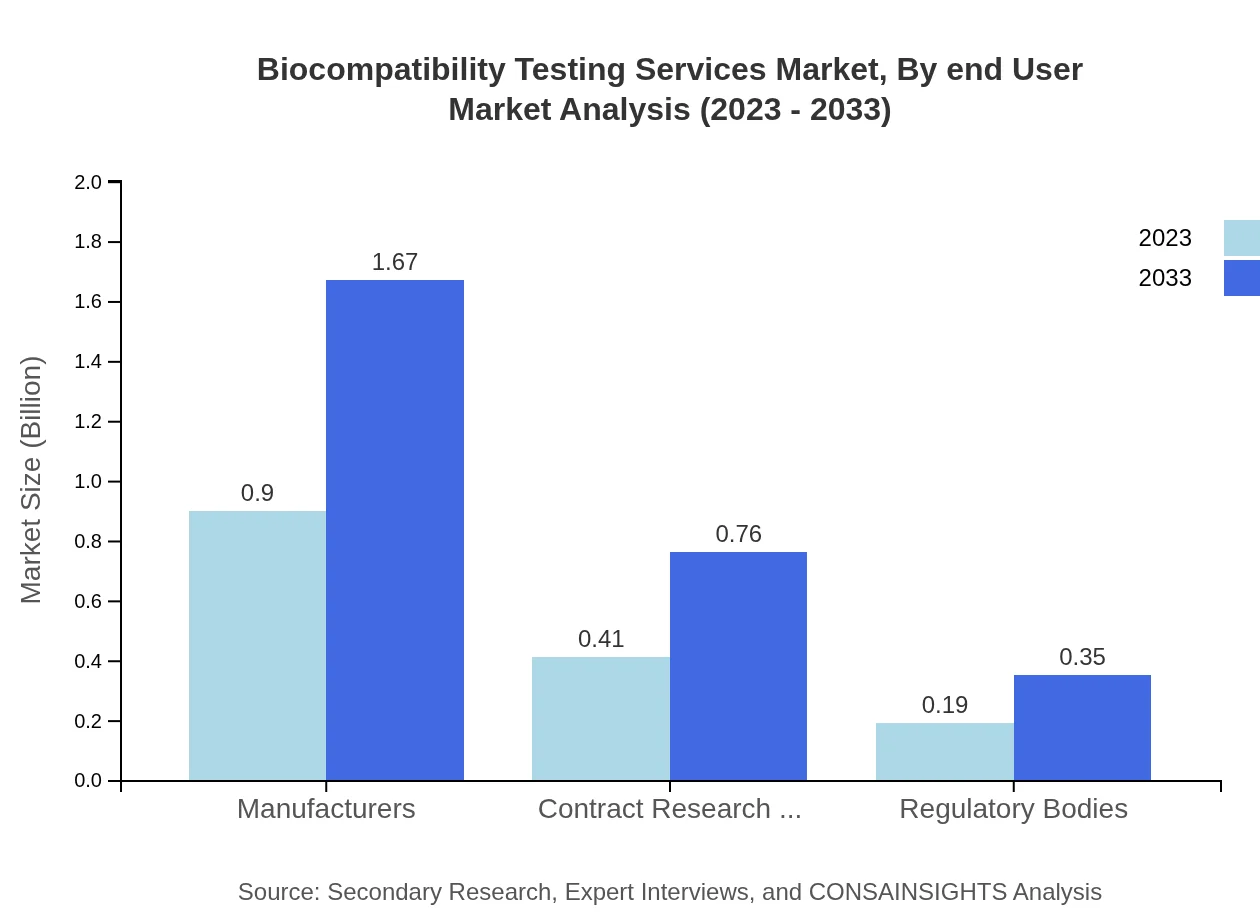
The Biocompatibility Testing Services Market, segmented by service type, highlights that manufacturers hold the largest market share, expected to grow from $0.90 billion in 2023 to $1.67 billion by 2033, capturing 60.2% of the market. CROs follow behind, with an increasing market size from $0.41 billion to $0.76 billion, thereby maintaining 27.35% market share. Regulatory bodies account for $0.19 billion, growing to $0.35 billion, capturing 12.45%. This segmentation showcases the reliance on established manufacturers and CROs for accurate testing services.
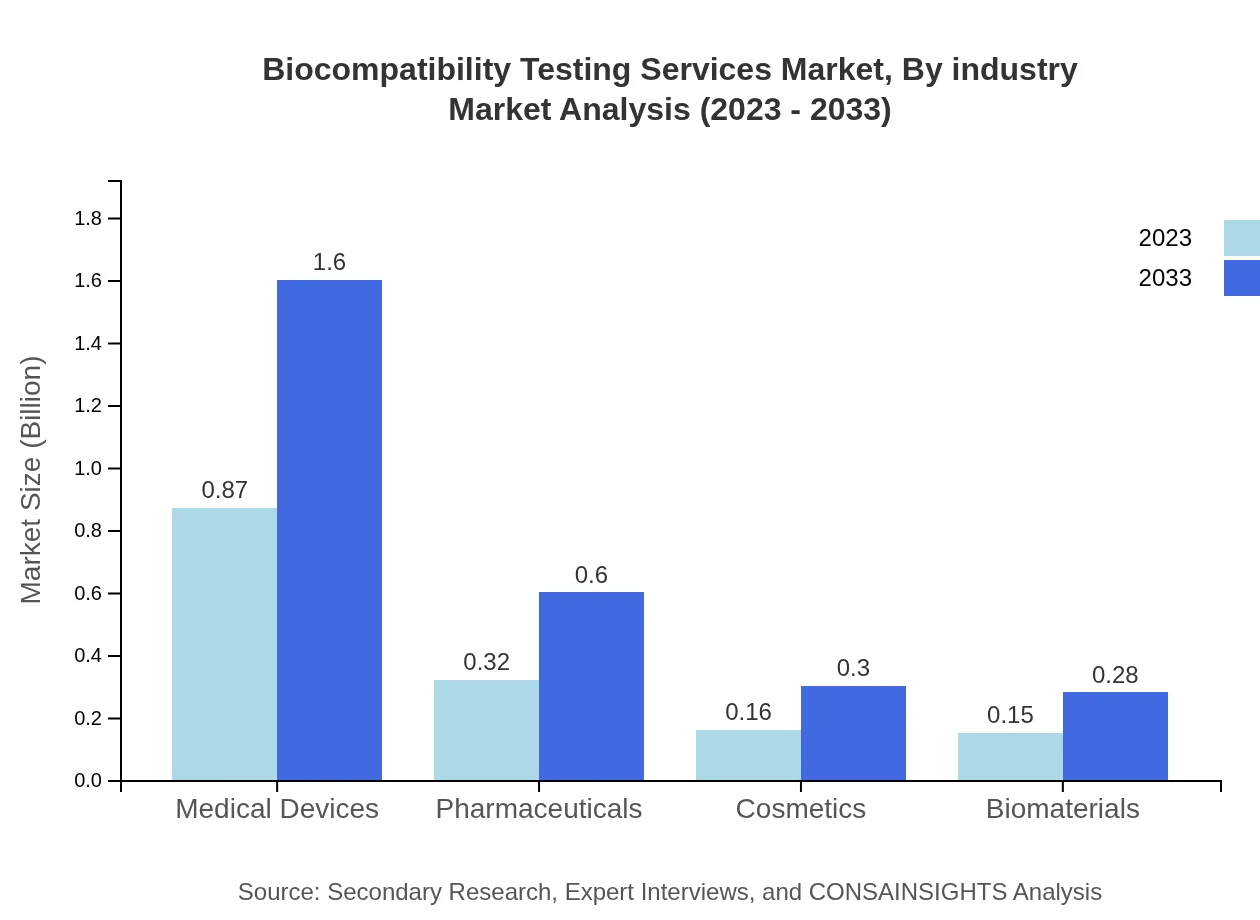
Biocompatibility Testing Services Market Analysis By Industry
In the Biocompatibility Testing Services Market, industry segmentation indicates that medical devices dominate with a market size rising from $0.87 billion in 2023 to $1.60 billion by 2033, accounting for a 57.69% share. Pharmaceuticals will see growth from $0.32 billion to $0.60 billion for a 21.43% share, while cosmetics contribute $0.16 billion to $0.30 billion and biomaterials progress from $0.15 billion to $0.28 billion, each capturing around 10% shares.
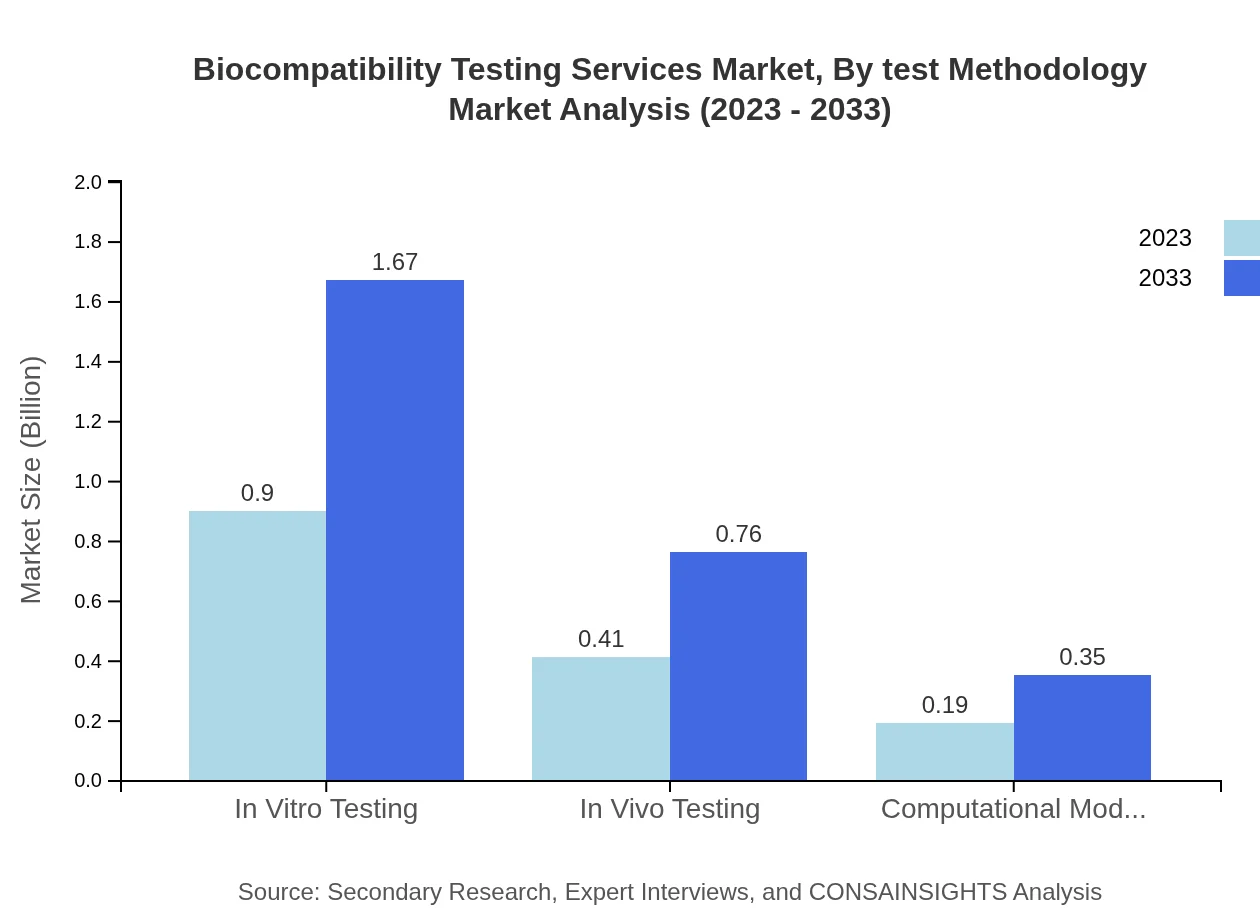
Biocompatibility Testing Services Market Analysis By Test Methodology
The testing methodology segment reveals that In Vitro Testing leads with a sizable market share, estimated at $0.90 billion growing to $1.67 billion, and accounting for 60.2%. In Vivo Testing grows from $0.41 billion to $0.76 billion, representing 27.35%. Computational Modeling also maintains a steady growth from $0.19 billion to $0.35 billion, capturing 12.45% market share, indicating a trend towards more technologically advanced testing methodologies.
Biocompatibility Testing Services Market Analysis By End User
The end-user analysis shows that major players in the medical device sector leverage biocompatibility testing extensively, with a market size of $2.00 billion in 2023 projected to reach $3.50 billion in 2033. Pharmaceuticals will extensively utilize these services, with the market expanding from $0.40 billion to $0.80 billion. The cosmetics sector is expected to see utilization increasing from $0.20 billion to $0.30 billion, showcasing the diverse applications of biocompatibility testing across sectors.
Biocompatibility Testing Services Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biocompatibility Testing Services Industry
Eurofins Scientific:
Eurofins Scientific is a global group of laboratories known for its commitment to quality and expertise in biocompatibility testing and analytical services, catering to the pharmaceutical and medical device sectors.Charles River Laboratories:
Charles River is a prominent provider of early-stage contract research services, offering comprehensive biocompatibility testing to support their clients in accelerating the development and approval of new pharmaceutical and biotechnology products.NAMSA:
Named as a leader in biocompatibility testing, NAMSA specializes in preclinical and clinical research services, helping manufacturers bring safe and effective medical devices to market.TÜV Rheinland:
TÜV Rheinland is a major player in the field, providing testing and certification to ensure products meet global standards for quality and safety, including those in biocompatibility.We're grateful to work with incredible clients.









FAQs
What is the market size of biocompatibility Testing Services?
The biocompatibility testing services market is currently valued at approximately $1.5 billion and is projected to grow at a CAGR of 6.2% over the next decade. This growth reflects rising demand from various industries for safety and compliance testing of materials.
What are the key market players or companies in this biocompatibility Testing Services industry?
Key players in the biocompatibility testing services industry include leading manufacturers, contract research organizations (CROs), and regulatory bodies. These entities are essential for driving standards and innovations in testing methodologies across various sectors.
What are the primary factors driving the growth in the biocompatibility Testing Services industry?
The growth in the biocompatibility testing services industry is propelled by increasing health regulations, a burgeoning medical devices market, and advancements in pharmaceuticals. Innovations in testing technologies and rising consumer awareness around product safety further enhance market expansion.
Which region is the fastest Growing in the biocompatibility Testing Services?
Among global regions, North America is the fastest-growing market for biocompatibility testing services, with expected growth from $0.54 billion in 2023 to $1.00 billion by 2033. Asia-Pacific also shows significant growth potential during this period.
Does ConsaInsights provide customized market report data for the biocompatibility Testing Services industry?
Yes, ConsaInsights offers tailored market report data based on specific client needs within the biocompatibility testing services sector. We analyze industry trends, segment data, and regional insights to provide actionable intelligence.
What deliverables can I expect from this biocompatibility Testing Services market research project?
Upon completion of the biocompatibility testing services market research project, clients can expect a comprehensive report detailing market size, growth forecasts, competitive landscape, segment analysis, and regional insights tailored to strategic planning.
What are the market trends of biocompatibility Testing Services?
Current trends in the biocompatibility testing services market include increased adoption of in vitro testing methods, enhanced focus on regulatory compliance, and heightened investment in R&D. The pharmaceutical and medical device sectors are particularly influential in shaping these trends.