Biologic Response Modifiers Market Report
Published Date: 31 January 2026 | Report Code: biologic-response-modifiers
Biologic Response Modifiers Market Size, Share, Industry Trends and Forecast to 2033
This report covers comprehensive insights into the Biologic Response Modifiers market, examining market dynamics, trends, forecasts (2023-2033), and detailed analyses by region, segment, and leading players in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
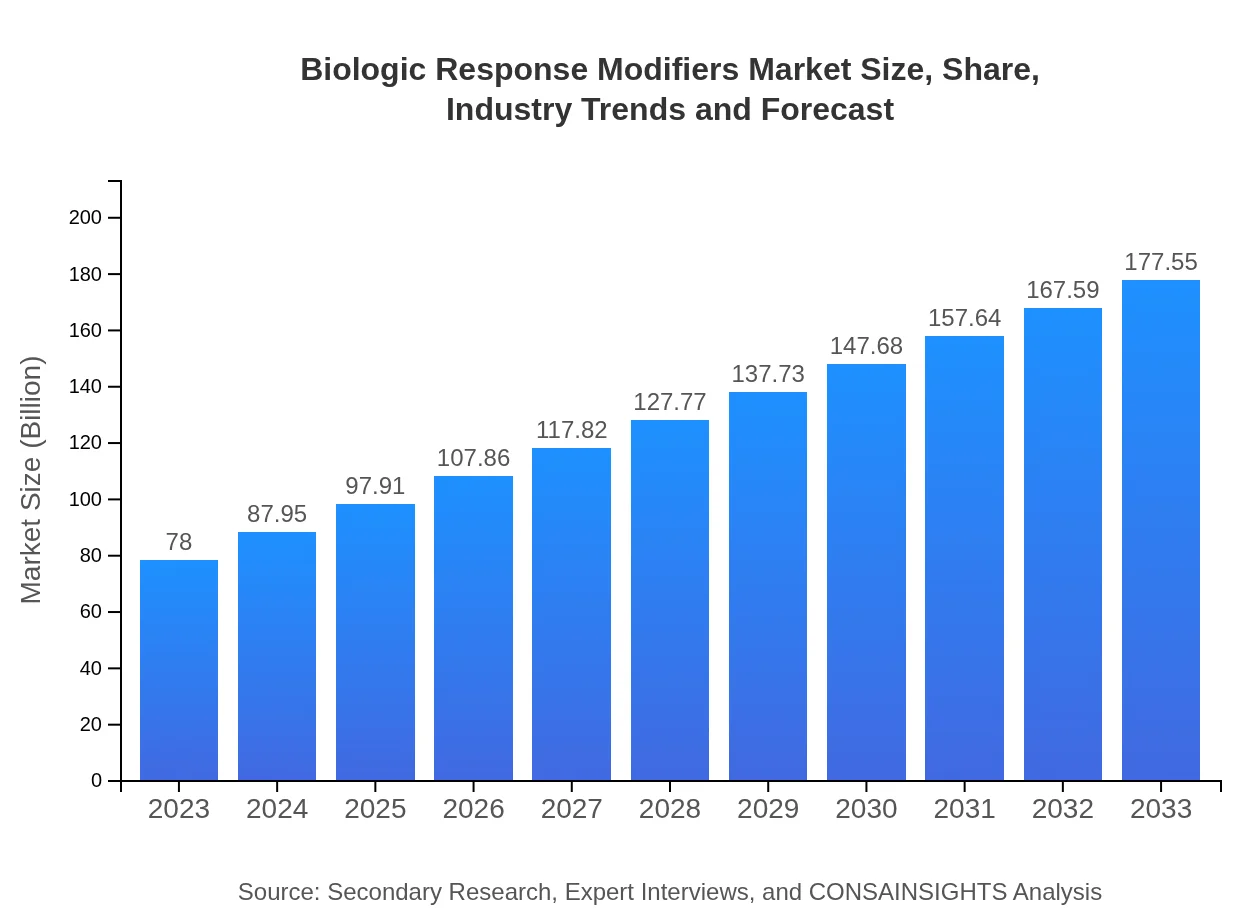
| 2023 Market Size | $78.00 Billion |
| CAGR (2023-2033) | 8.3% |
| 2033 Market Size | $177.55 Billion |
| Top Companies | Roche Holding AG, Amgen Inc., AbbVie Inc., Johnson & Johnson, Novartis AG |
| Last Modified Date | 31 January 2026 |
Biologic Response Modifiers Market Overview
Customize Biologic Response Modifiers Market Report market research report
- ✔ Get in-depth analysis of Biologic Response Modifiers market size, growth, and forecasts.
- ✔ Understand Biologic Response Modifiers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biologic Response Modifiers
What is the Market Size & CAGR of Biologic Response Modifiers Market in 2033?
Biologic Response Modifiers Industry Analysis
Biologic Response Modifiers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biologic Response Modifiers Market Analysis Report by Region
Europe Biologic Response Modifiers Market Report:
Europe's market is expected to grow from $19.46 billion in 2023 to $44.30 billion in 2033, driven by robust healthcare infrastructure and a growing elderly population requiring innovative therapies.Asia Pacific Biologic Response Modifiers Market Report:
In Asia Pacific, the market is expected to expand from $15.80 billion in 2023 to $35.97 billion in 2033, reflecting an increasing investment in healthcare infrastructure and access to advanced therapies, while government initiatives support research and development.North America Biologic Response Modifiers Market Report:
North America is the largest market, with an estimated value of $27.99 billion in 2023, projected to reach $63.70 billion by 2033, due to favorable reimbursement scenarios and the presence of major pharmaceutical companies.South America Biologic Response Modifiers Market Report:
The South American market will grow from $7.01 billion in 2023 to $15.96 billion by 2033, supported by rising awareness of biologic therapies and improving healthcare systems.Middle East & Africa Biologic Response Modifiers Market Report:
In the Middle East and Africa, the market will increase from $7.74 billion in 2023 to $17.61 billion by 2033, influenced by enhancing healthcare services and a rising prevalence of chronic diseases.Tell us your focus area and get a customized research report.
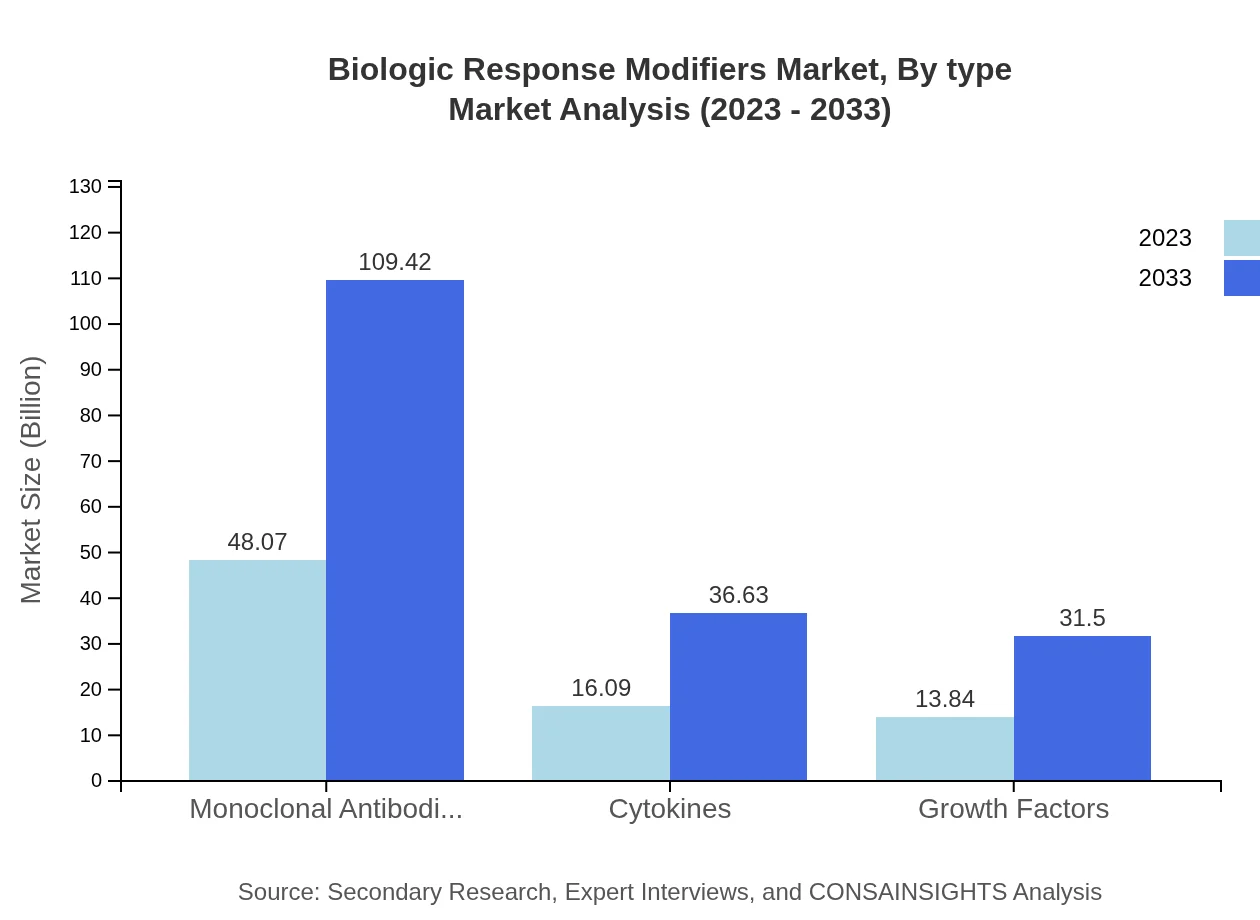
Biologic Response Modifiers Market Analysis By Type
The market is segmented into monoclonal antibodies, cytokines, and growth factors. Monoclonal antibodies dominate the market due to their application in cancer treatment, growing from $48.07 billion in 2023 to $109.42 billion by 2033, holding a major share of 61.63%. Cytokines are expected to grow from $16.09 billion to $36.63 billion, with a share of 20.63%, while growth factors increase from $13.84 billion to $31.50 billion, representing 17.74% market share.
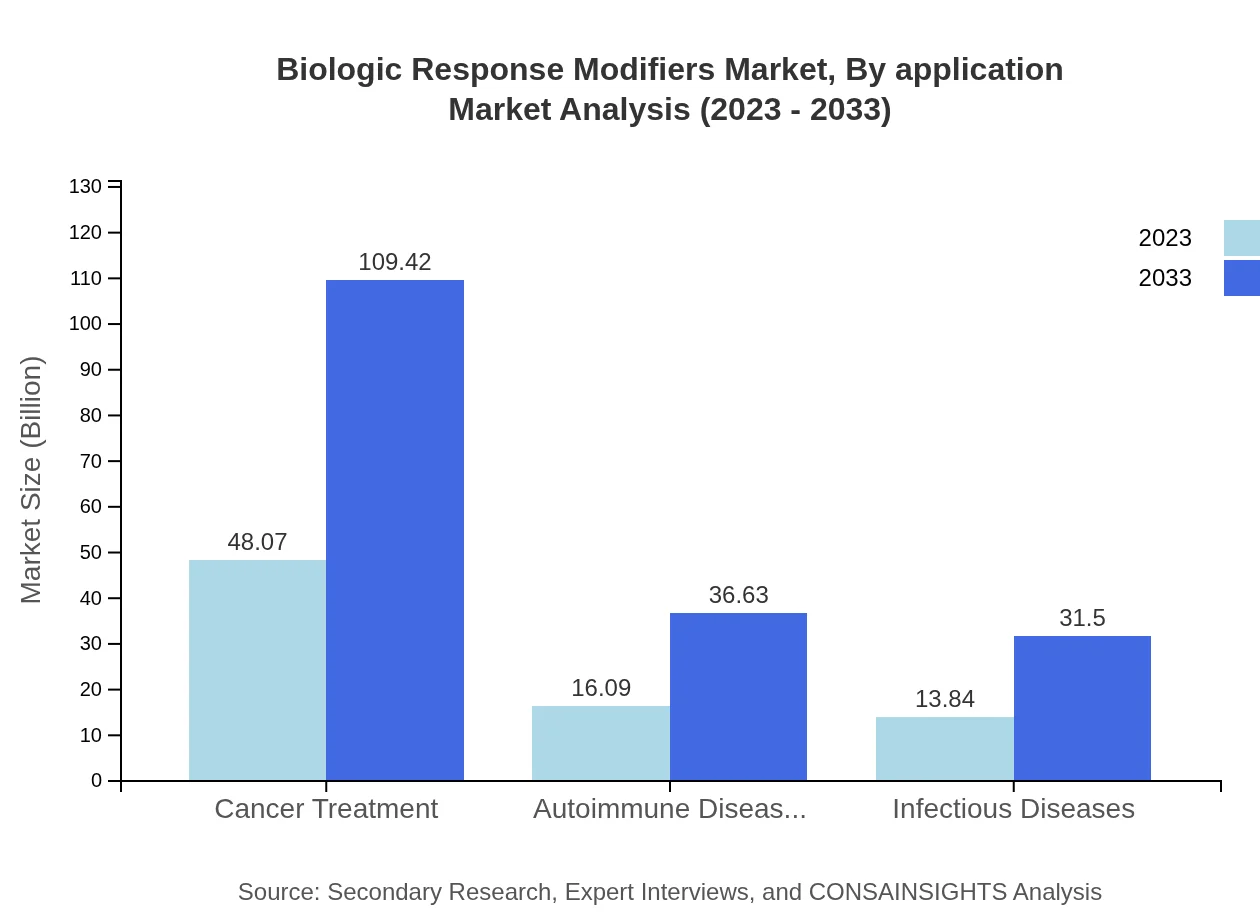
Biologic Response Modifiers Market Analysis By Application
This segment highlights key applications in cancer treatment, autoimmune diseases, and infectious diseases. Cancer treatment accounts for the largest market share and is projected to grow from $48.07 billion to $109.42 billion. Autoimmune diseases will see growth from $16.09 billion to $36.63 billion, while infectious diseases will grow from $13.84 billion to $31.50 billion.
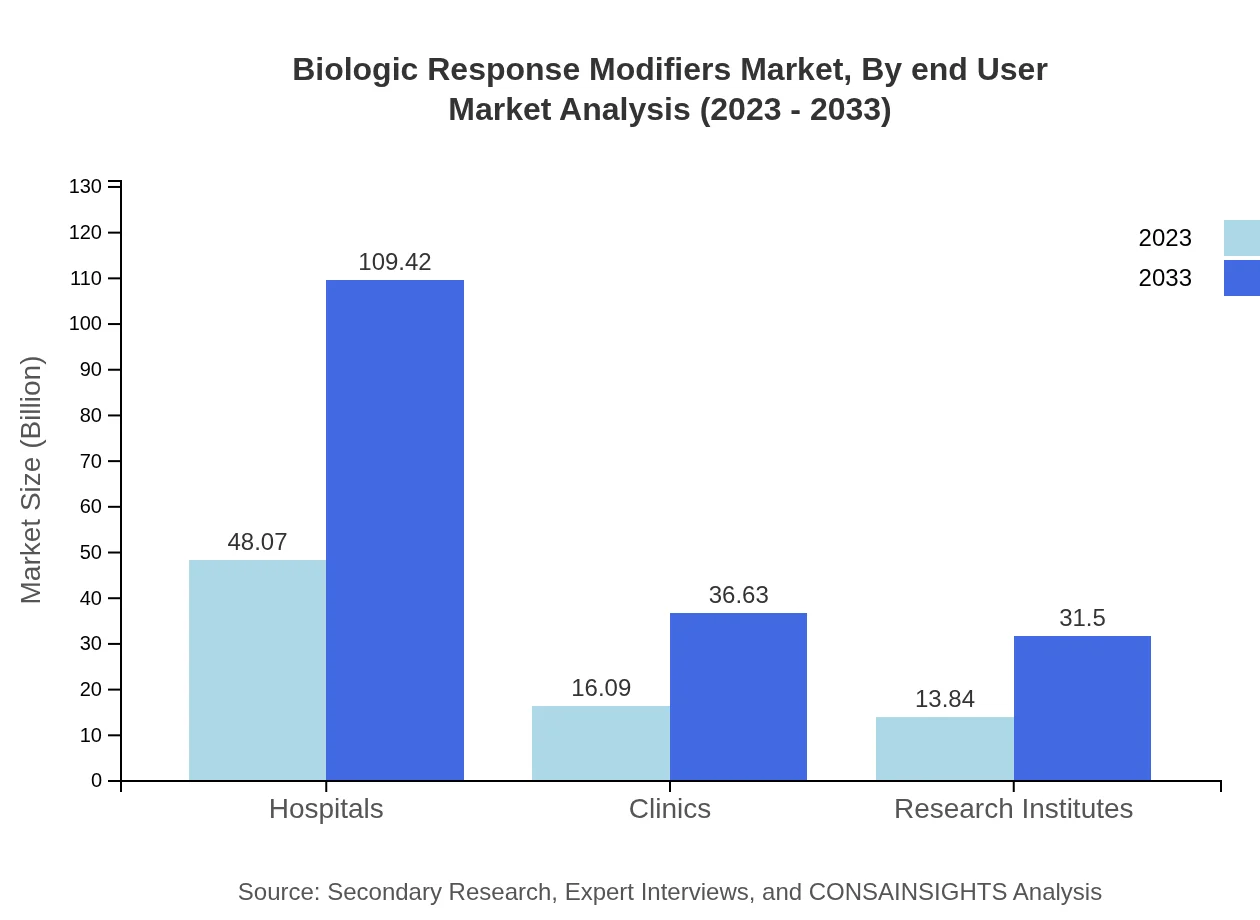
Biologic Response Modifiers Market Analysis By End User
End-users include hospitals, clinics, and research institutes. Hospitals lead with a market size of $48.07 billion in 2023, growing to $109.42 billion. Clinics are projected to expand from $16.09 billion to $36.63 billion and research institutes from $13.84 billion to $31.50 billion, reflecting increased research and clinical practices.
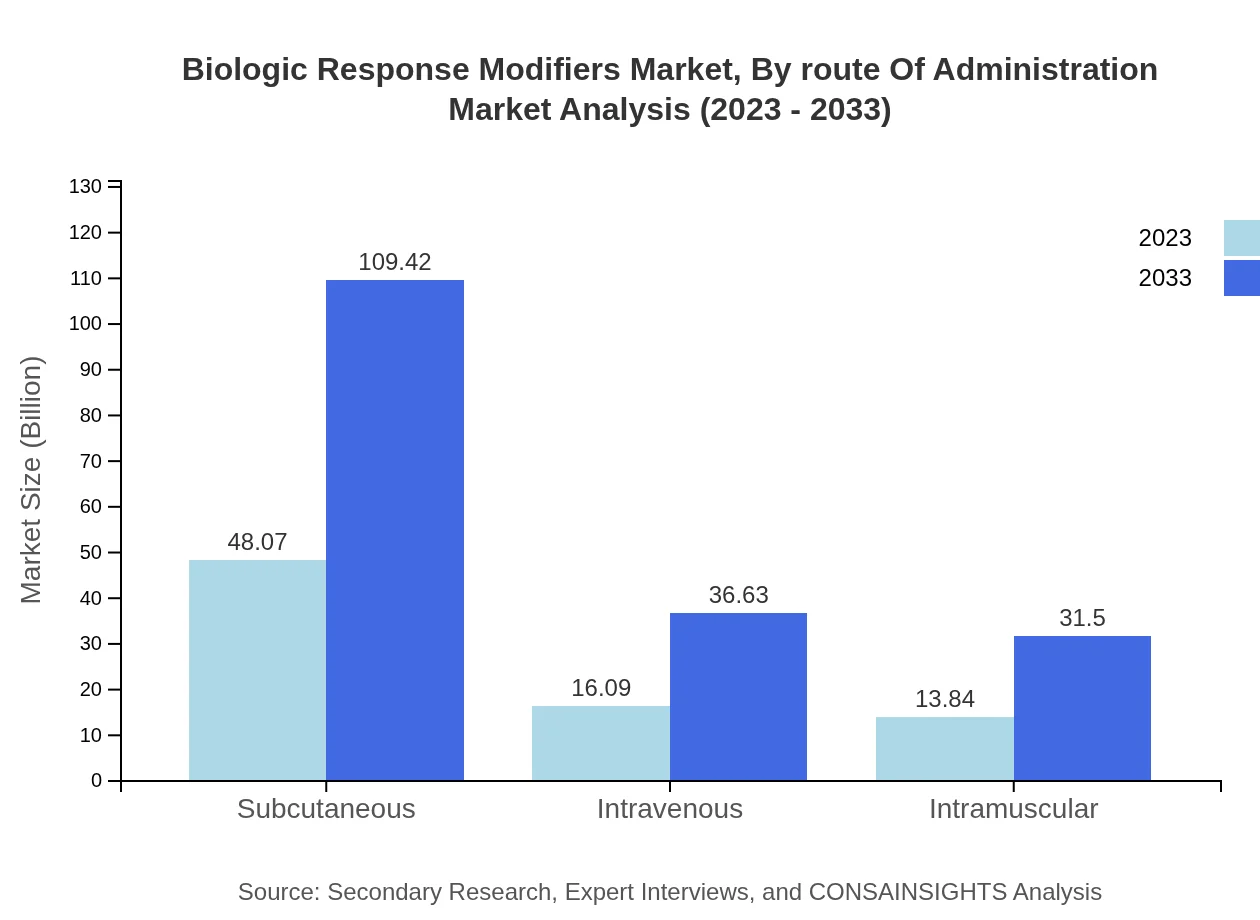
Biologic Response Modifiers Market Analysis By Route Of Administration
The route of administration is segmented into subcutaneous, intravenous, and intramuscular. Subcutaneous methods dominate with $48.07 billion in 2023, reaching $109.42 billion. Intravenous routes will grow from $16.09 billion to $36.63 billion, while intramuscular options will see an increase from $13.84 billion to $31.50 billion.
Biologic Response Modifiers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biologic Response Modifiers Industry
Roche Holding AG:
A leading player in the biotech sector, Roche has been pivotal in developing innovative monoclonal antibody therapies, focusing on oncology and immunology.Amgen Inc.:
Known for its biotechnology innovations, Amgen specializes in developing therapeutic proteins and monoclonal antibodies for serious diseases.AbbVie Inc.:
AbbVie is a key market player with a strong portfolio of immunology and oncology drugs, focusing on biologic therapies that enhance patient outcomes.Johnson & Johnson:
With a robust pipeline in immunology and oncology, Johnson & Johnson is committed to expanding its biologic portfolio through innovation.Novartis AG:
This company plays a crucial role in the biologic response modifiers market, focusing on treatments for various chronic and autoimmune diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of biologic Response Modifiers?
The global Biologic Response Modifiers market is valued at approximately $78 billion in 2023, with a projected CAGR of 8.3% through 2033, indicating robust growth driven by advancements in biotechnology and increasing prevalence of diseases.
What are the key market players or companies in this biologic Response Modifiers industry?
Key players in the Biologic Response Modifiers market include major pharmaceutical companies like Amgen, Roche, Johnson & Johnson, AbbVie, and Merck. These companies significantly influence market dynamics through innovation, strategic collaborations, and product development.
What are the primary factors driving the growth in the biologic Response Modifiers industry?
Growth in the biologic-response-modifiers industry is driven by factors such as increasing incidence of chronic diseases, advancements in biotechnology, heightened R&D investments, and rising healthcare expenditures worldwide, supporting market expansion.
Which region is the fastest Growing in the biologic Response Modifiers?
The fastest-growing region in the Biologic Response Modifiers market is North America, projected to grow from $27.99 billion in 2023 to $63.70 billion by 2033, supported by strong healthcare infrastructure and increasing adoption of biologic therapies.
Does ConsaInsights provide customized market report data for the biologic Response Modifiers industry?
Yes, ConsaInsights offers customized market report data tailored to specific client needs in the biologic-response-modifiers industry, ensuring insights align with unique research objectives and market requirements.
What deliverables can I expect from this biologic Response Modifiers market research project?
From the biologic-response-modifiers market research project, you can expect comprehensive reports including market size and dynamics, competitive landscape analysis, regional insights, segment breakdowns, and future trends within the industry.
What are the market trends of biologic Response Modifiers?
Current trends in the biologic-response-modifiers market include growing preference for monoclonal antibodies, increased usage of cytokines, and innovations in drug delivery methods, reflecting the ongoing evolution and modernization of therapeutic approaches.