Biologics Safety Testing Market Report
Published Date: 31 January 2026 | Report Code: biologics-safety-testing
Biologics Safety Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Biologics Safety Testing market, covering market trends, sizes, forecasts up to 2033, and insights into segments and regional performance.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
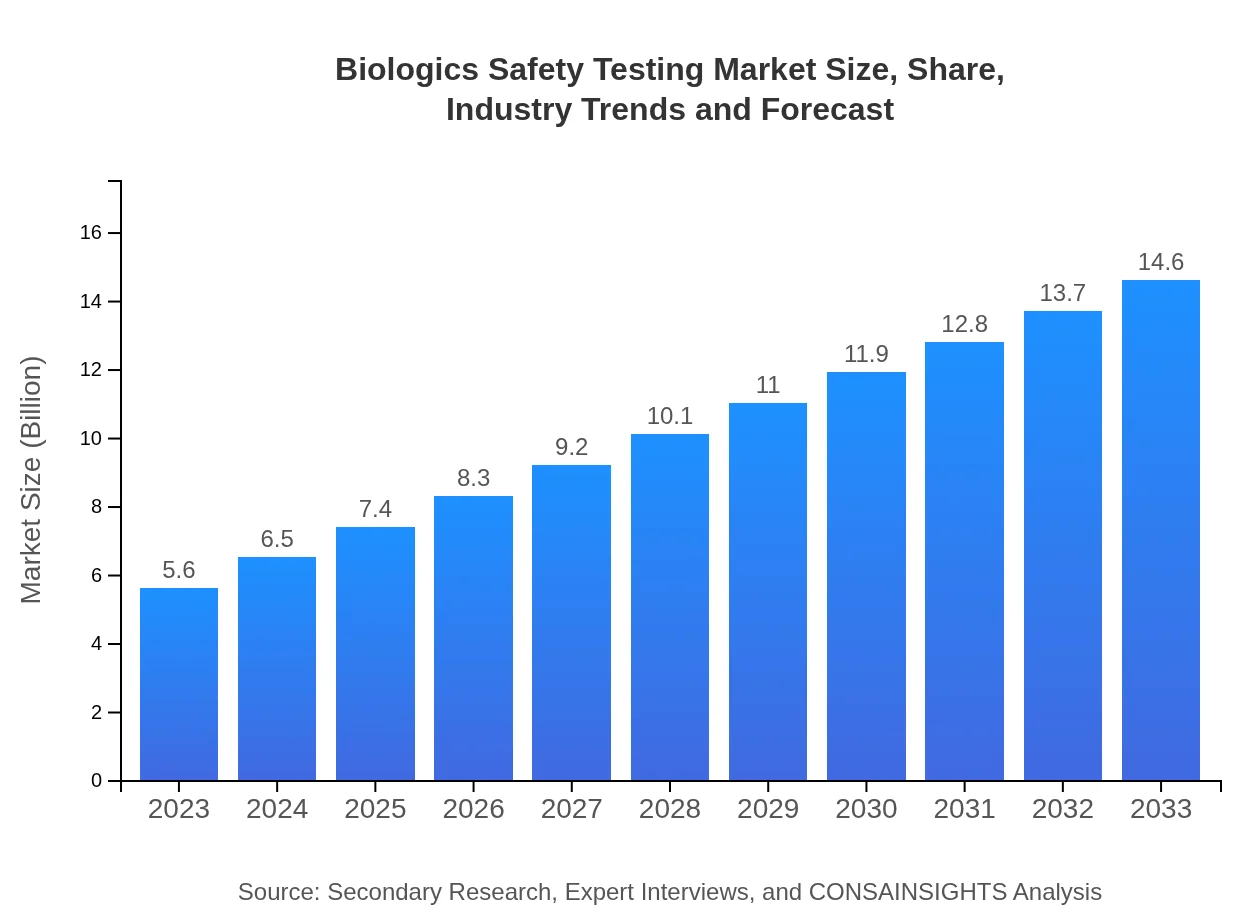
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.7% |
| 2033 Market Size | $14.60 Billion |
| Top Companies | Charles River Laboratories, Eurofins Scientific, SGS SA, LabCorp |
| Last Modified Date | 31 January 2026 |
Biologics Safety Testing Market Overview
Customize Biologics Safety Testing Market Report market research report
- ✔ Get in-depth analysis of Biologics Safety Testing market size, growth, and forecasts.
- ✔ Understand Biologics Safety Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biologics Safety Testing
What is the Market Size & CAGR of Biologics Safety Testing market in 2023?
Biologics Safety Testing Industry Analysis
Biologics Safety Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biologics Safety Testing Market Analysis Report by Region
Europe Biologics Safety Testing Market Report:
Europe’s market is expected to rise from $1.78 billion in 2023 to $4.64 billion by 2033, influenced by continuous advancements in biologics and a focus on innovative safety testing methodologies to comply with stringent EU regulations.Asia Pacific Biologics Safety Testing Market Report:
The Asia-Pacific region is anticipated to grow from $1.04 billion in 2023 to $2.73 billion by 2033, representing a notable increase driven by growing biopharmaceutical sectors and increasing investments in healthcare infrastructure. The demand for quality assurance testing in biological products is escalating as countries in this region impose stricter regulations.North America Biologics Safety Testing Market Report:
North America accounts for the largest market share, with a projected increase from $1.97 billion in 2023 to $5.13 billion by 2033. The robust regulatory framework and the presence of major pharmaceutical and biotechnology companies contribute to the steady growth in this region.South America Biologics Safety Testing Market Report:
In South America, the Biologics Safety Testing market is expected to expand from $0.35 billion in 2023 to $0.92 billion by 2033. The growth is driven by the increasing collaborations between local manufacturers and international companies aimed at ensuring compliance with global safety standards.Middle East & Africa Biologics Safety Testing Market Report:
In the Middle East and Africa, the market size is projected to grow from $0.45 billion in 2023 to $1.18 billion by 2033. There is a growing emphasis on safety testing in response to the increasing prevalence of biologics being produced in this region, encouraging investments in safety practices.Tell us your focus area and get a customized research report.
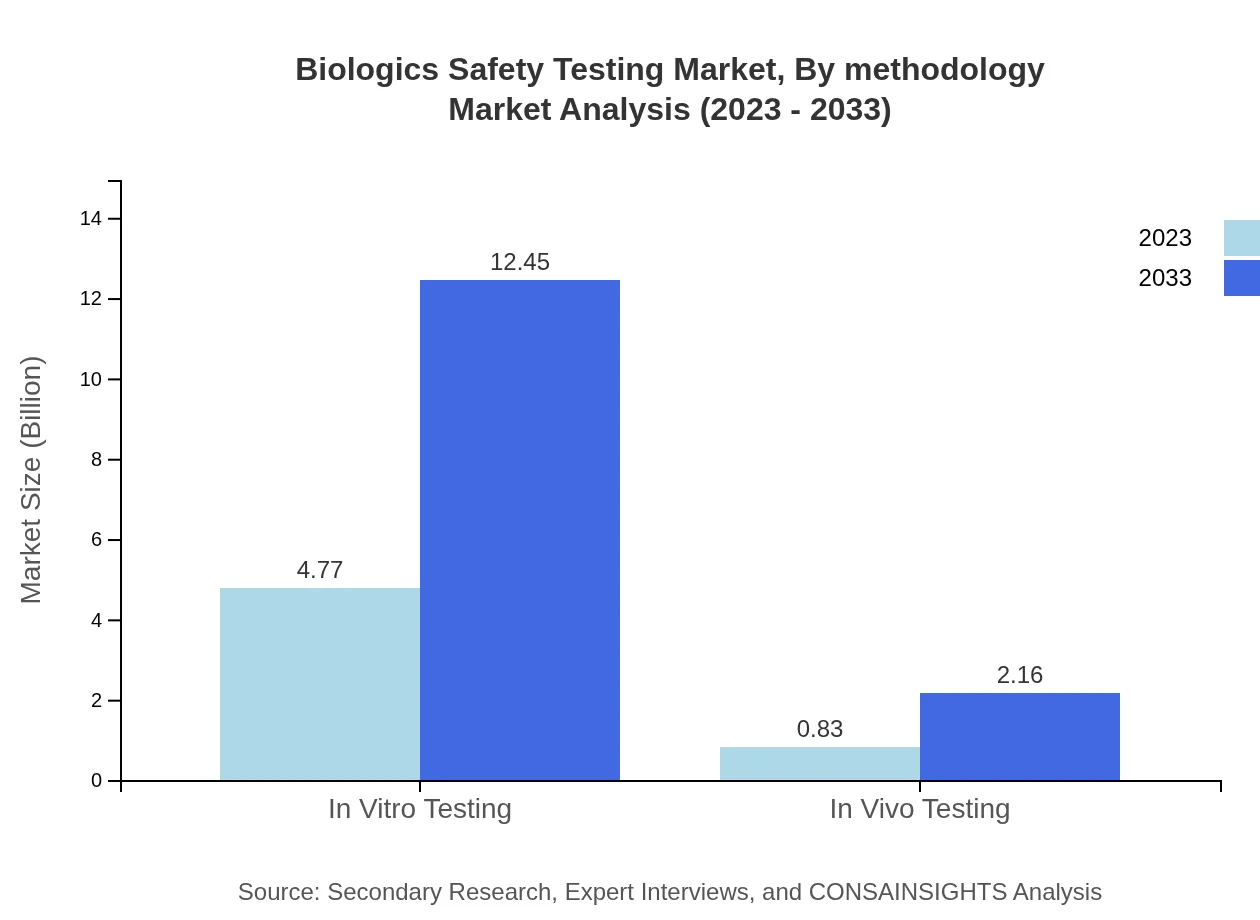
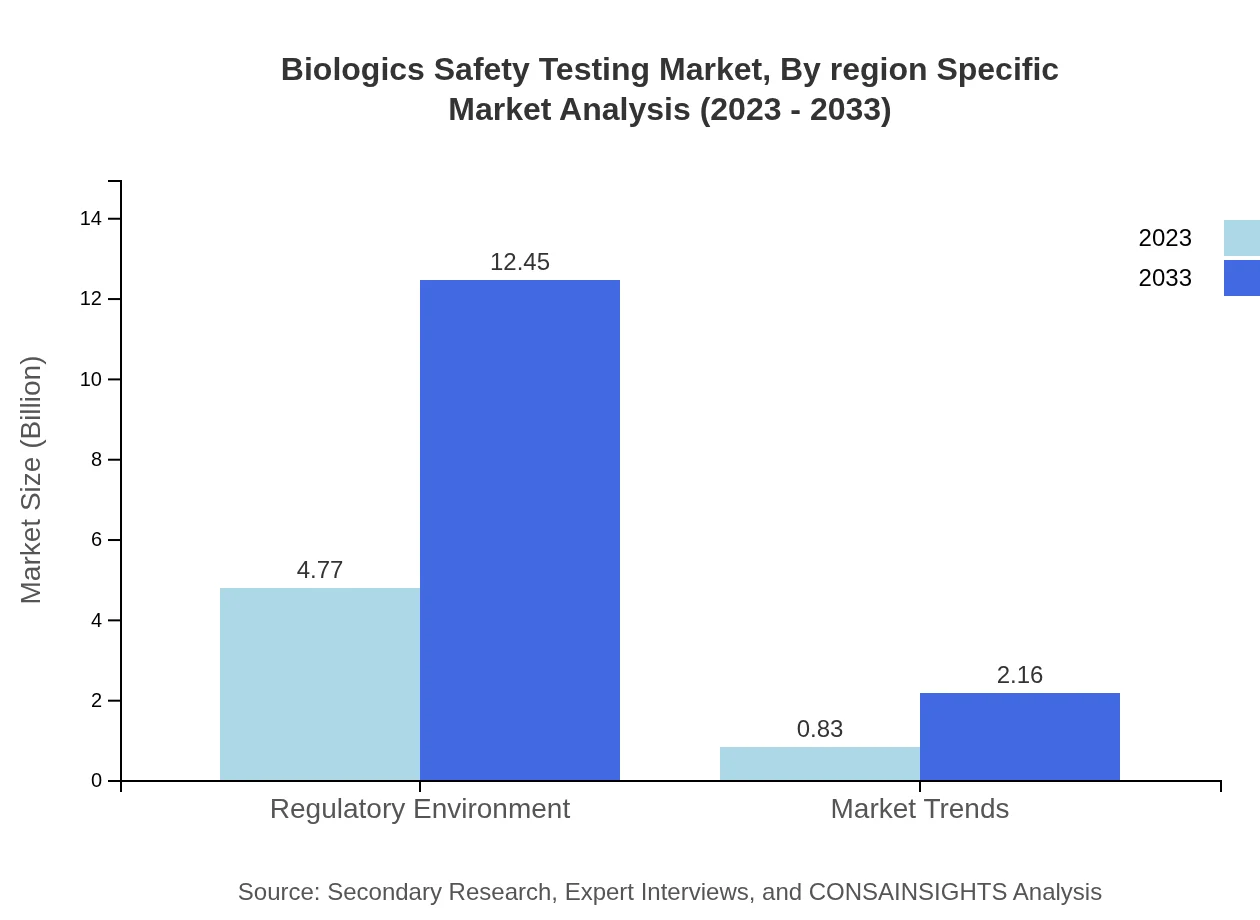
Biologics Safety Testing Market Analysis By Methodology
The segment analysis highlights a significant reliance on in vitro testing, which dominates the market with a size of $4.77 billion in 2023, expected to grow to $12.45 billion by 2033. In vivo testing, though smaller, shows substantial growth potential, expanding from $0.83 billion to $2.16 billion in the same period.
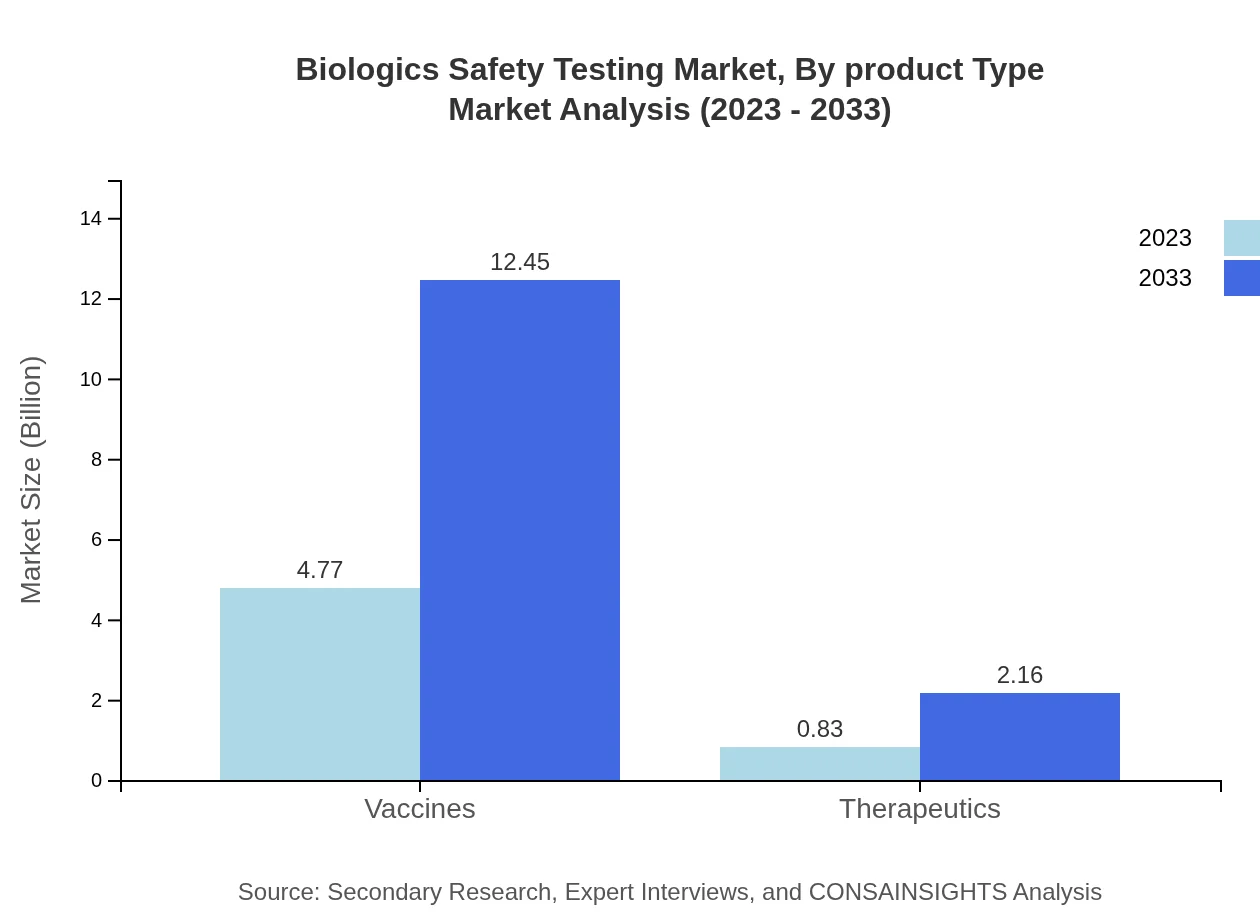
Biologics Safety Testing Market Analysis By Product Type
Vaccines make up a substantial portion of the market, growing from $4.77 billion in 2023 to $12.45 billion by 2033. Therapeutics and diagnostics are also critical, showing a rising demand as they play a vital role in compliance with safety standards.
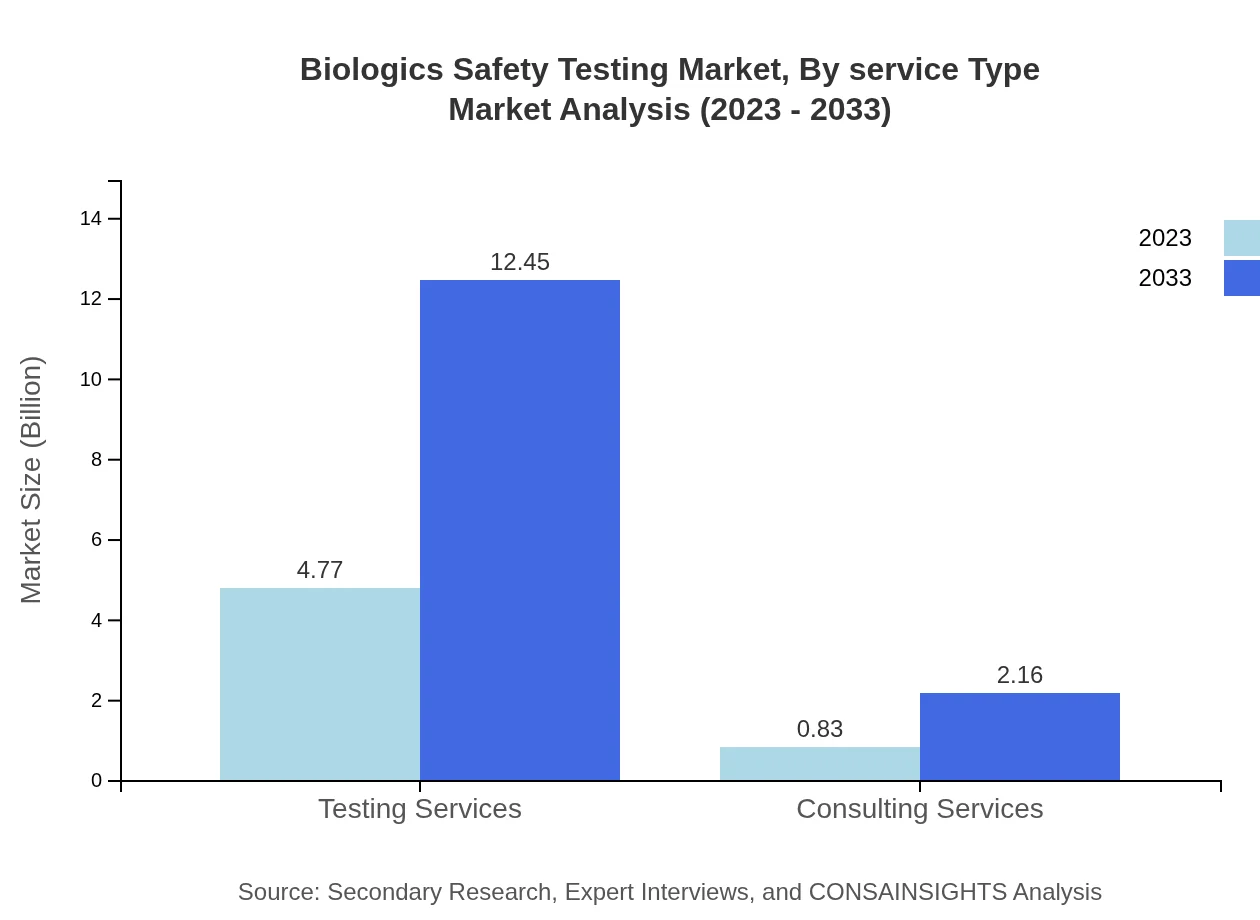
Biologics Safety Testing Market Analysis By Service Type
Testing services encompass the majority of the market share, with significant contributions from consulting services. Testing services are set to increase from $4.77 billion in 2023 to $12.45 billion in 2033, asserting their importance in the industry.
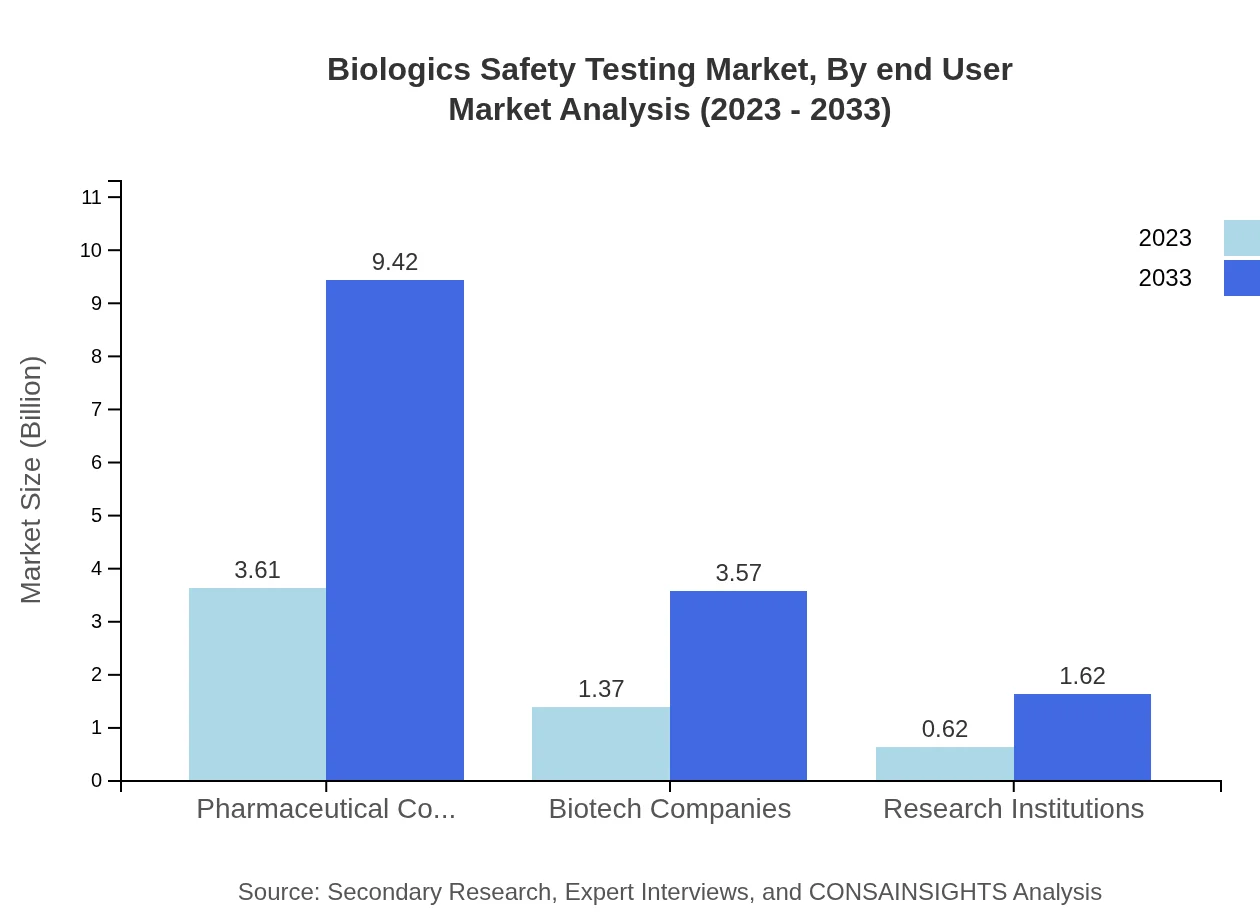
Biologics Safety Testing Market Analysis By End User
Pharmaceutical and biotech companies lead the end-user segment, with a market size of $3.61 billion expected to reach $9.42 billion by 2033. Research institutions also play a significant role, with revenues projected to rise from $0.62 billion to $1.62 billion.
Biologics Safety Testing Market Analysis By Region Specific
Various regional factors uniquely influence market growth. North America drives innovation due to mature infrastructure, while Asia Pacific benefits from rapid expansion in production capabilities. Regulatory challenges in Europe also indicate essential regional comparisons.
Biologics Safety Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biologics Safety Testing Industry
Charles River Laboratories:
A leading provider of preclinical and clinical laboratory services for the biopharmaceutical industry, Charles River is essential in providing comprehensive safety testing services.Eurofins Scientific:
Specializing in bioanalytical testing, Eurofins offers a range of services necessary for biologics safety testing, helping pharmaceutical companies ensure their products meet regulatory compliance.SGS SA:
With a global presence, SGS provides inspection, verification, testing, and certification services, including crucial testing for biologics to ensure compliance and safety.LabCorp:
LabCorp plays a significant role in laboratory testing and provides comprehensive solutions for biologics safety assessment, supporting the development pipeline of many medications.We're grateful to work with incredible clients.









FAQs
What is the market size of biologics Safety Testing?
The biologics safety testing market is valued at $5.6 billion in 2023 and is expected to grow at a CAGR of 9.7% through 2033, reflecting a robust demand for safety testing in biologics.
What are the key market players or companies in this biologics Safety Testing industry?
Key players in the biologics safety testing industry include large pharmaceutical companies and specialized biotech firms, which dominate the market with advanced technologies and comprehensive testing services.
What are the primary factors driving the growth in the biologics Safety Testing industry?
Growth in the biologics safety testing industry is driven by increasing drug development, regulatory requirements for safety testing, and rising investments in biotechnology research and development.
Which region is the fastest Growing in the biologics Safety Testing?
North America is projected as the fastest-growing region in biologics safety testing, with the market growing from $1.97 billion in 2023 to $5.13 billion in 2033.
Does ConsaInsights provide customized market report data for the biologics Safety Testing industry?
Yes, ConsaInsights offers customized market reports tailored to specific client needs in the biologics safety testing industry, ensuring relevant and actionable insights.
What deliverables can I expect from this biologics Safety Testing market research project?
Deliverables may include detailed market analysis, segmentation insights, competitive landscape assessment, growth forecasts, and strategic recommendations for stakeholders in the biologics safety testing sector.
What are the market trends of biologics Safety Testing?
Current trends include increasing demand for in vitro testing, advancements in regulatory guidelines, and a shift towards more efficient and cost-effective safety testing methodologies.