Biomarker Technologies Market Report
Published Date: 31 January 2026 | Report Code: biomarker-technologies
Biomarker Technologies Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Biomarker Technologies market, exploring insights on market size, trends, and forecasts from 2023 to 2033. Key areas include industry analysis, regional analysis, market segmentation, and profiles of global leaders in the field.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
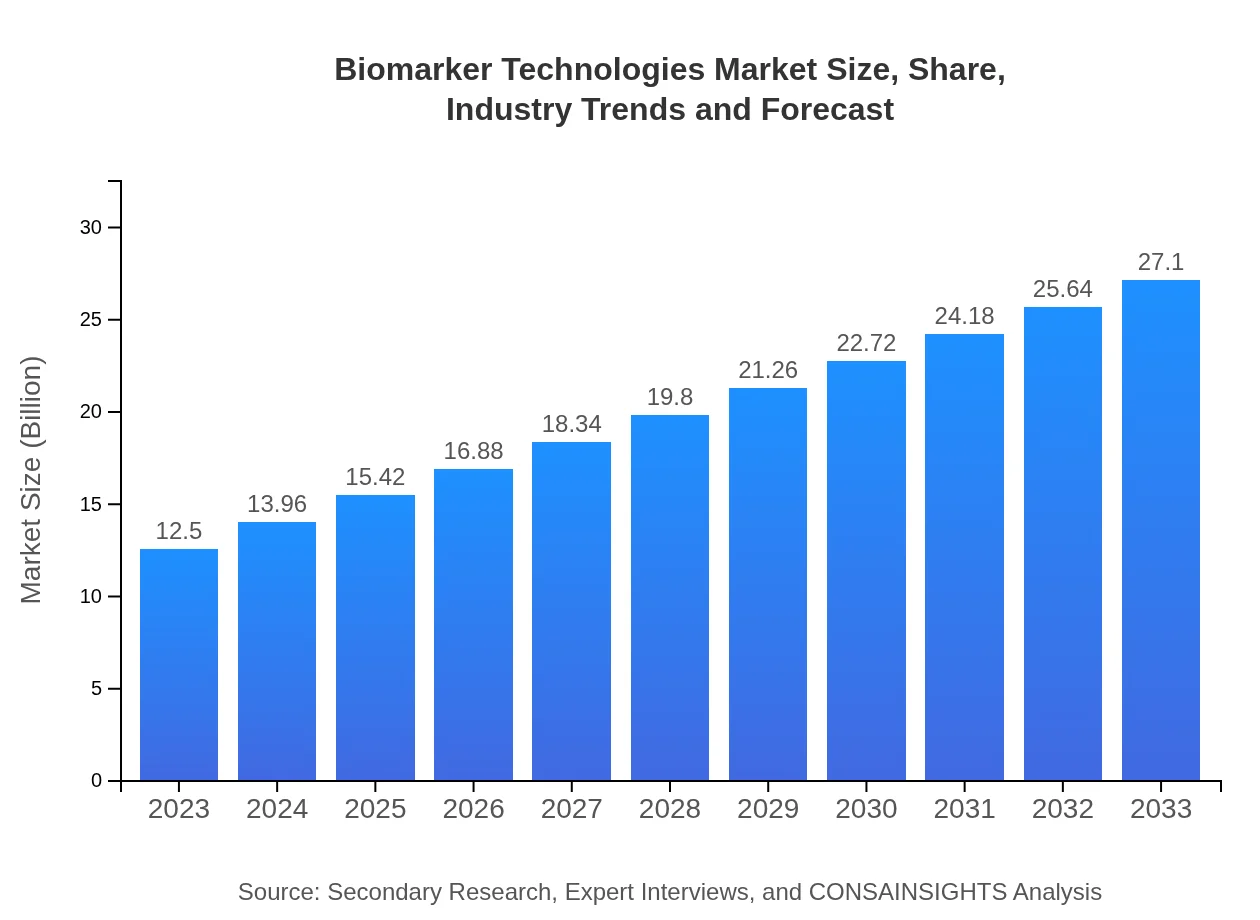
| 2023 Market Size | $12.50 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $27.10 Billion |
| Top Companies | Roche, Thermo Fisher Scientific, Abbott Laboratories, Illumina |
| Last Modified Date | 31 January 2026 |
Biomarker Technologies Market Overview
Customize Biomarker Technologies Market Report market research report
- ✔ Get in-depth analysis of Biomarker Technologies market size, growth, and forecasts.
- ✔ Understand Biomarker Technologies's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biomarker Technologies
What is the Market Size & CAGR of Biomarker Technologies market in 2023?
Biomarker Technologies Industry Analysis
Biomarker Technologies Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biomarker Technologies Market Analysis Report by Region
Europe Biomarker Technologies Market Report:
Europe's market size is estimated at $3.64 billion in 2023, projected to reach $7.89 billion by 2033. The European region benefits from strong regulatory frameworks supporting drug development and biomarker integration, coupled with high patient awareness and favorable reimbursement policies, enhancing market growth.Asia Pacific Biomarker Technologies Market Report:
The Asia Pacific region, valued at $2.37 billion in 2023, is anticipated to reach $5.15 billion by 2033. The market growth is attributed to increasing investments in healthcare infrastructure and rising prevalence of diseases. Countries such as China and India are pivotal due to their large populations and rapid urbanization, which leads to higher disease incidence and demand for advanced diagnostic tools.North America Biomarker Technologies Market Report:
North America marks the largest market for Biomarker Technologies, with a valuation of $4.61 billion in 2023 and expected growth to $10 billion by 2033. This surge is supported by robust healthcare spending, high adoption rates of advanced technologies in hospitals, and the presence of major market players driving innovation.South America Biomarker Technologies Market Report:
In South America, the market is projected to grow from $0.41 billion in 2023 to $0.88 billion by 2033. The region is experiencing gradual improvements in healthcare systems and rising research initiatives. Increased awareness about precision medicine and biomarker applications are fueling this demand.Middle East & Africa Biomarker Technologies Market Report:
The Middle East and Africa region, valued at $1.47 billion in 2023, is likely to expand to $3.18 billion by 2033. Key factors include improving healthcare infrastructure, increased spending on healthcare R&D, and a growing emphasis on early disease detection and management solutions.Tell us your focus area and get a customized research report.
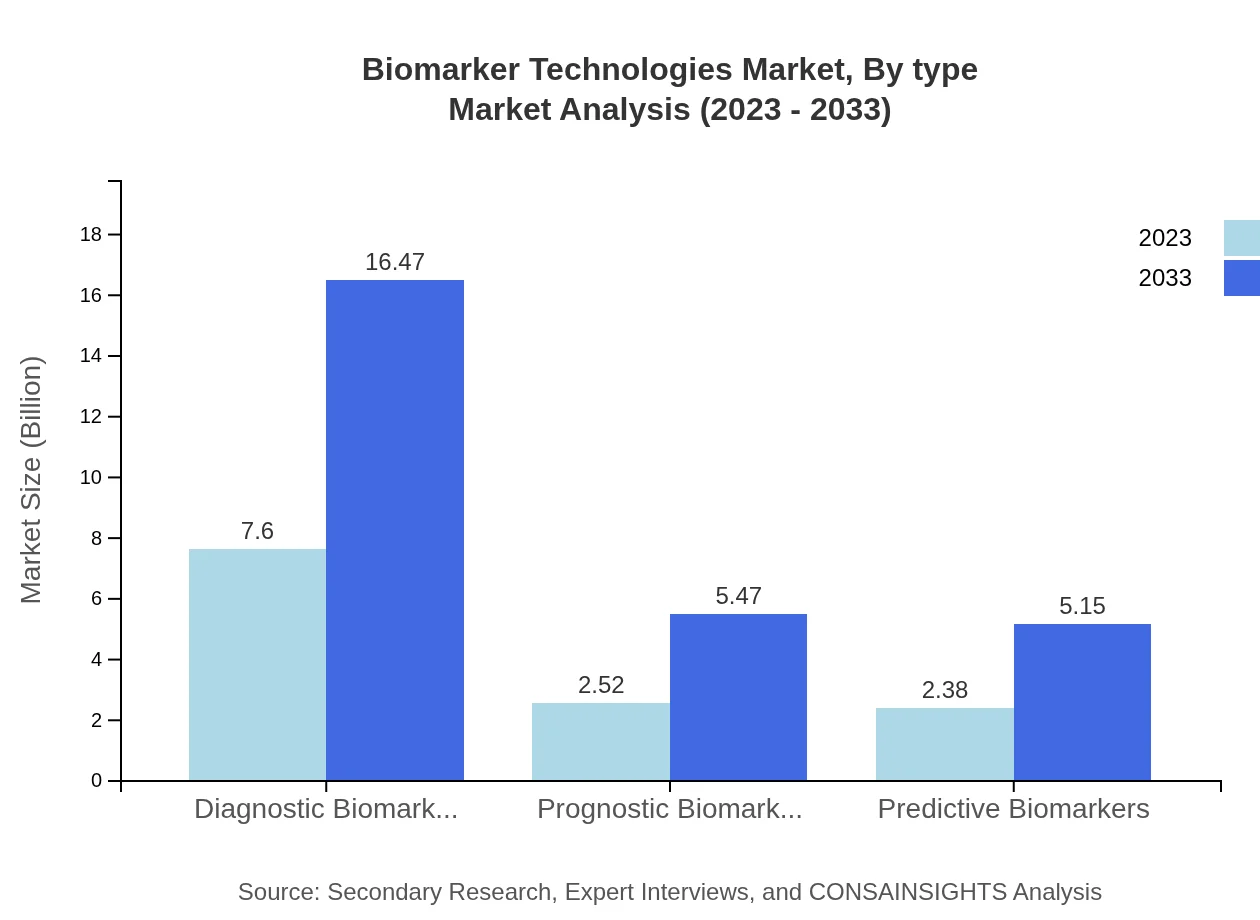
Biomarker Technologies Market Analysis By Type
In 2023, the market for Diagnostic Biomarkers is valued at $7.60 billion and is expected to grow to $16.47 billion by 2033, holding a market share of 60.79%. Prognostic and Predictive Biomarkers are also pertinent, with sizes of $2.52 billion (20.2% share) and $2.38 billion (19.01% share) respectively. The growth of diagnostic biomarkers is largely attributed to increasing applications in disease identification and monitoring.
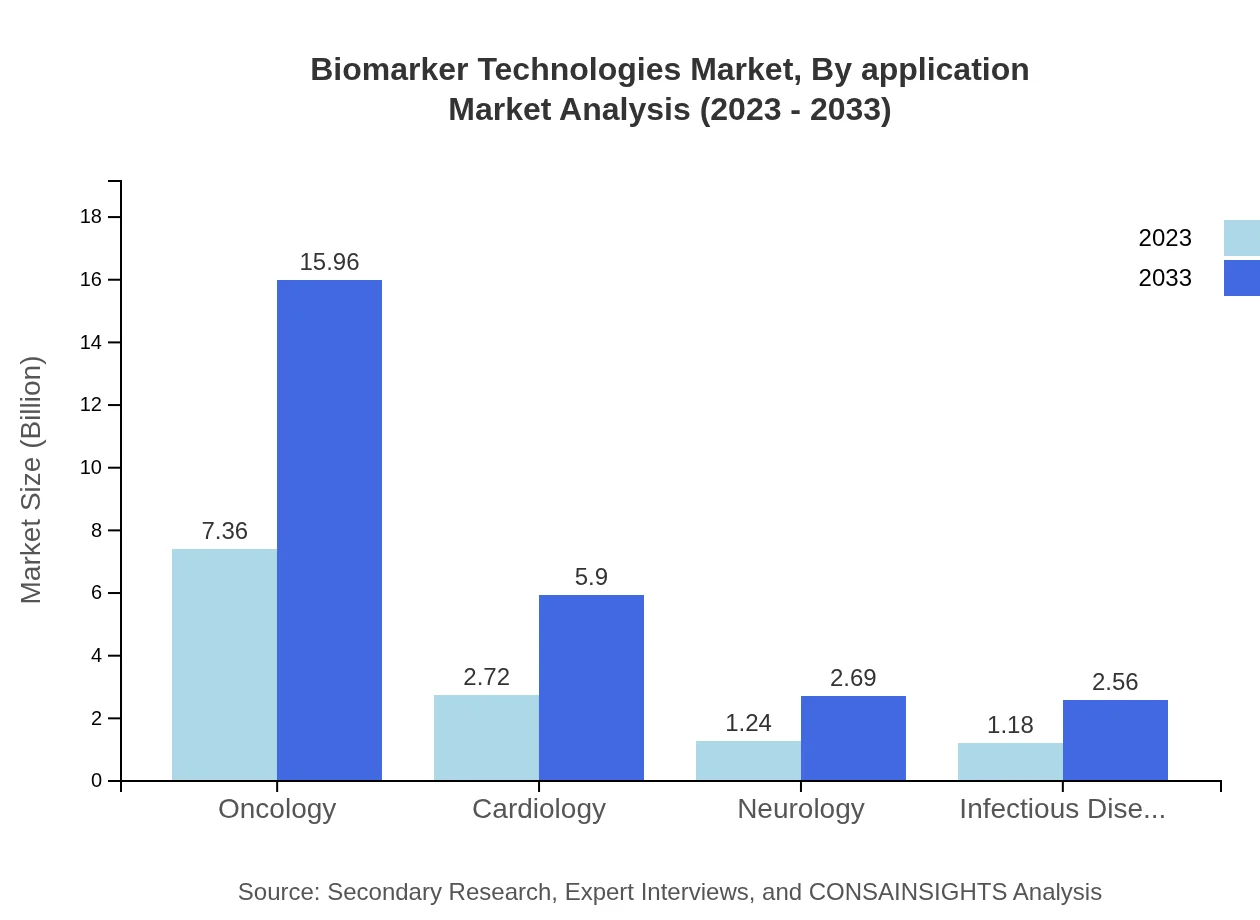
Biomarker Technologies Market Analysis By Application
Oncology leads the application segment, expected to grow from $7.36 billion in 2023 to $15.96 billion by 2033, capturing 58.88% of the market. Other significant applications are Cardiology and Neurology, projected to grow from $2.72 billion and $1.24 billion, respectively. The prioritization of oncology remains pivotal due to its inherent complexities and the need for tailored treatment strategies.
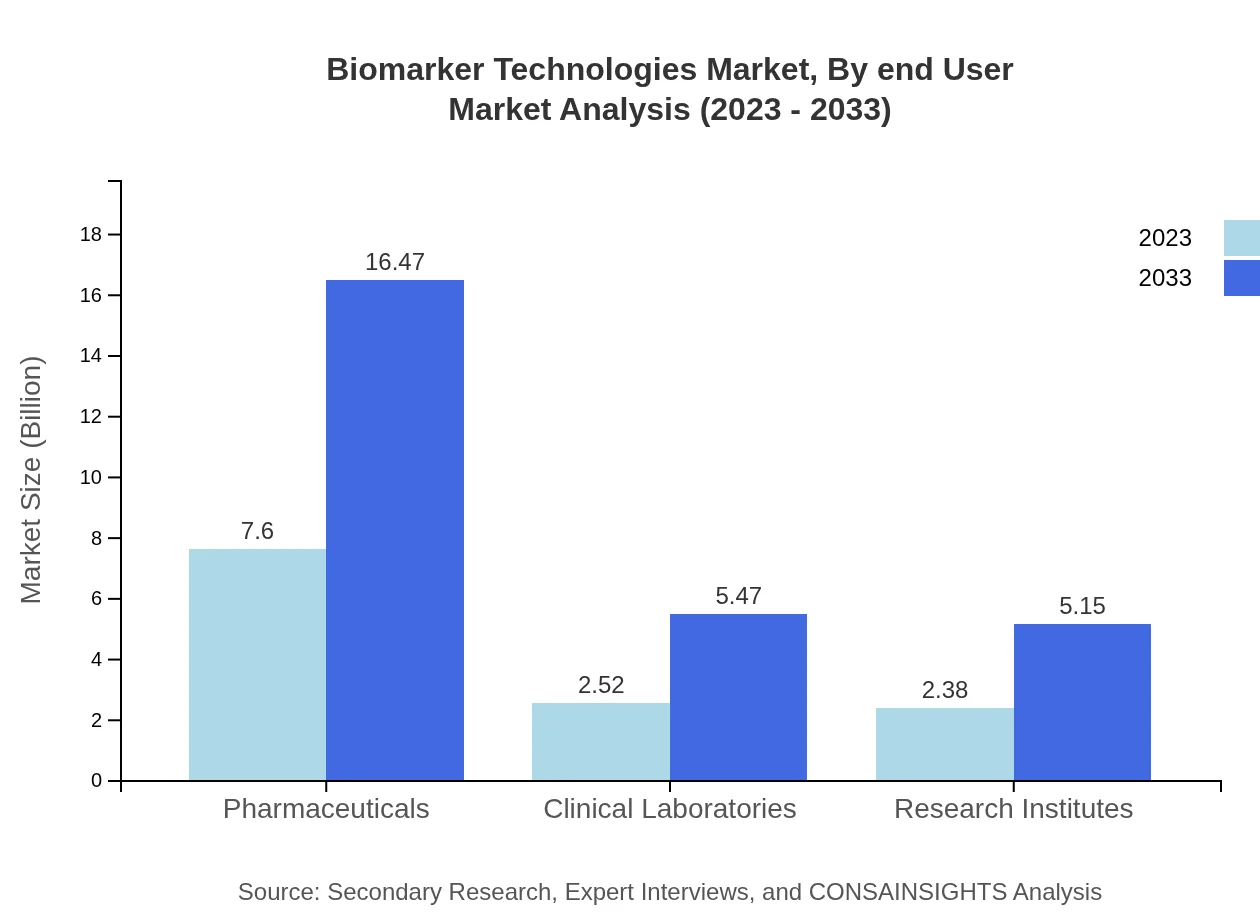
Biomarker Technologies Market Analysis By End User
Pharmaceutical companies dominate the end-user category, with a market size of $7.60 billion in 2023, projected to reach $16.47 billion by 2033, controlling 60.79% of the market. Clinical laboratories and Research Institutes are also key users, with sizes of $2.52 billion and $2.38 billion respectively, reflecting increased research and development activities.
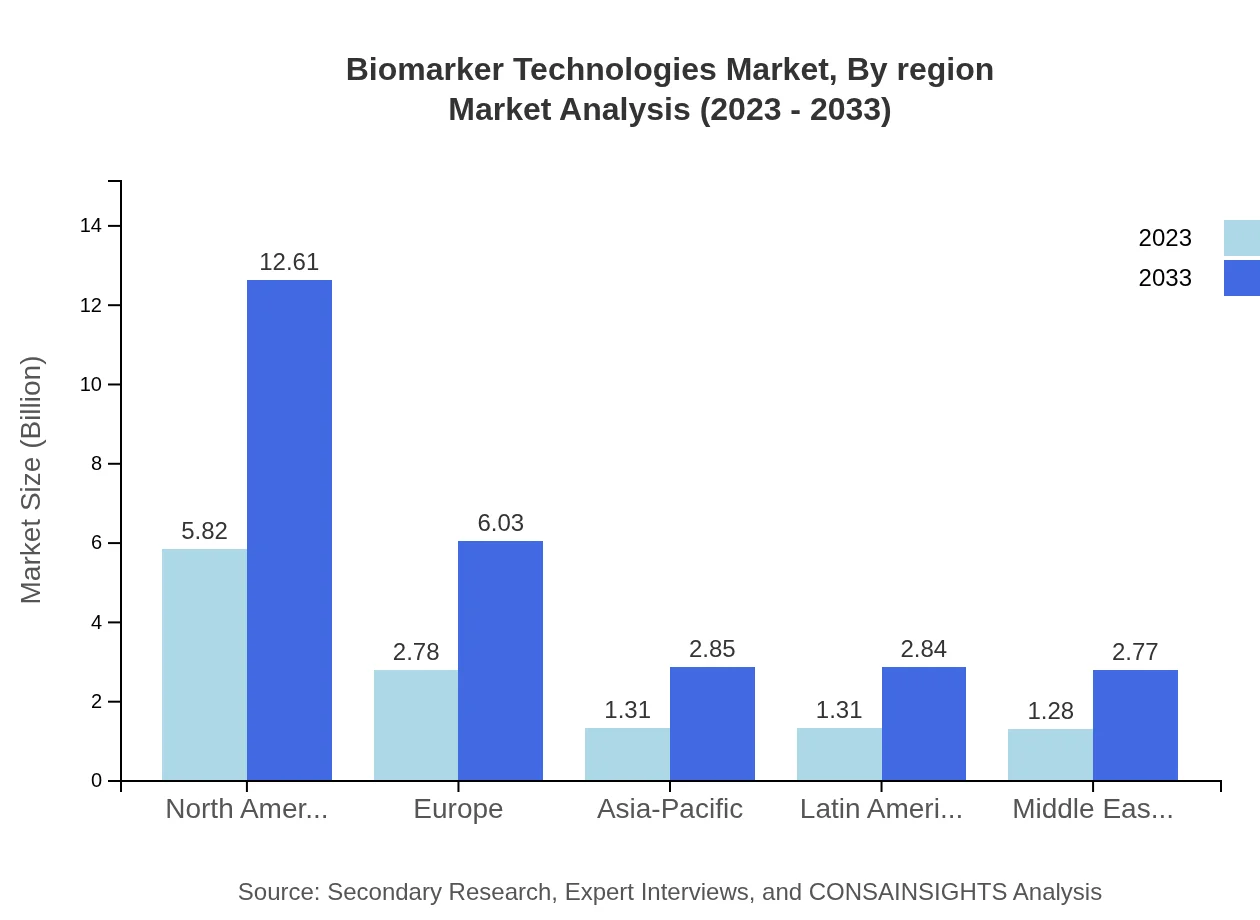
Biomarker Technologies Market Analysis By Region
Region-wise, North America leads with $5.82 billion in 2023, forecast to reach $12.61 billion by 2033 (46.54% share). Europe follows at $2.78 billion in 2023, and will grow to $6.03 billion by 2033 (22.24% share). Asia-Pacific and Latin America show promising growth trajectories, underpinned by increased healthcare investments and disease awareness.
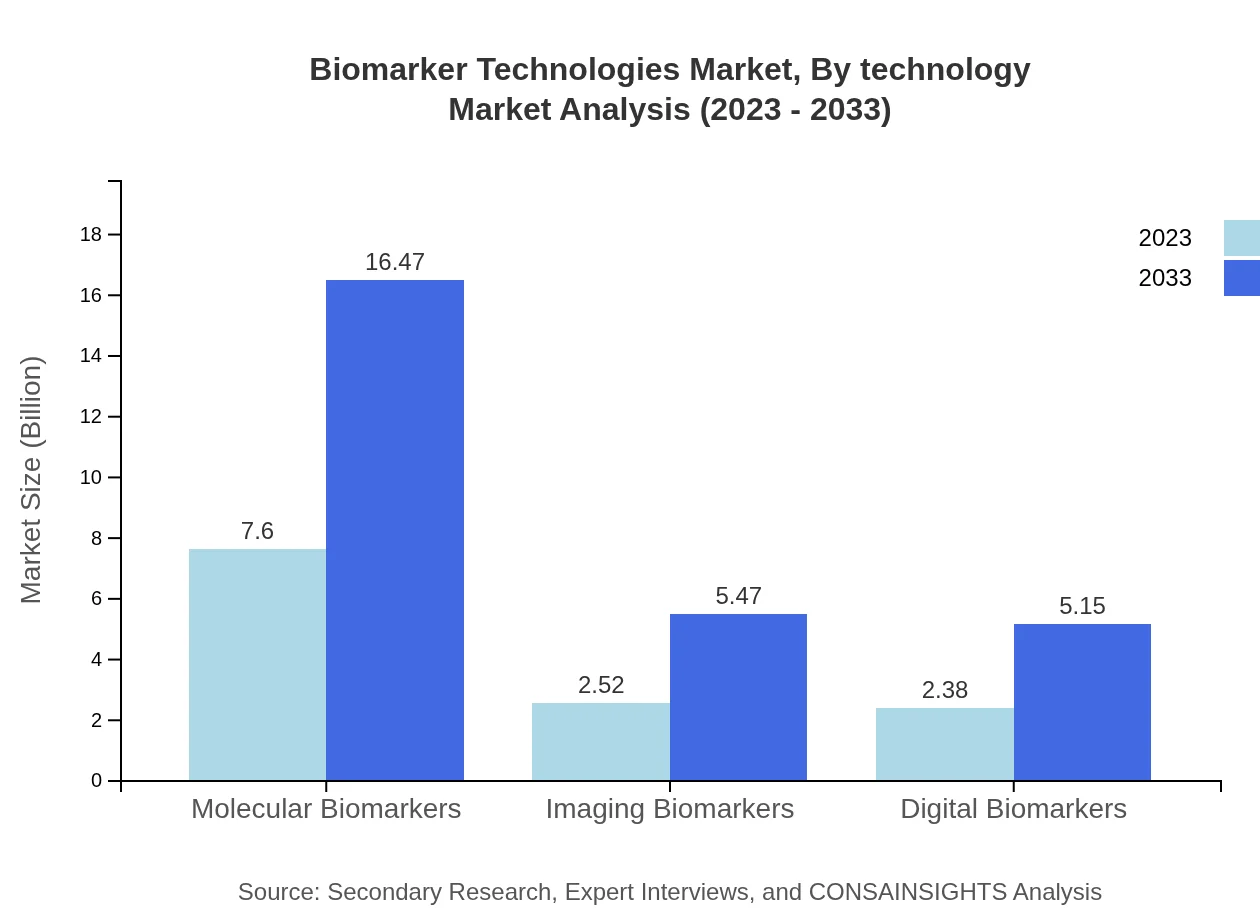
Biomarker Technologies Market Analysis By Technology
Technological advancements in genomics, proteomics, and imaging systems have significantly enhanced the Biomarker Technologies landscape. Novel technologies enabling liquid biopsies and mobile health applications are emerging trends. The technological shift towards automation and high-throughput screening methods is also making diagnostics more accessible and efficient.
Biomarker Technologies Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biomarker Technologies Industry
Roche:
Roche is a global leader in providing innovative diagnostic solutions, focusing on advanced biomarker technologies for oncology.Thermo Fisher Scientific:
Thermo Fisher Scientific offers a diverse range of biomarker assay technologies and instruments, aiding research and clinical applications.Abbott Laboratories:
Abbott Laboratories is known for its robust diagnostics portfolio, enhancing patient care through innovative biomarker tests.Illumina:
Illumina specializes in genomics and contributes significantly to the biomarker discovery process through next-generation sequencing platforms.We're grateful to work with incredible clients.









FAQs
What is the market size of biomarker Technologies?
The biomarker technologies market is projected to reach approximately $12.5 billion by 2033, growing at a CAGR of 7.8%. This growth is driven by the increasing demand for personalized medicine and advancements in diagnostic methods.
What are the key market players or companies in this biomarker Technologies industry?
Key players in the biomarker technologies industry include major biotechnology and pharmaceutical companies that focus on innovative diagnostic solutions, therapeutic biomarkers, and personalized medicine, contributing to the market's expansion.
What are the primary factors driving the growth in the biomarker Technologies industry?
Growth in the biomarker technologies industry is primarily driven by increased R&D activities, technological advancements, and the rising prevalence of chronic diseases, requiring innovative diagnostic and therapeutic solutions.
Which region is the fastest Growing in the biomarker technologies?
North America is expected to be the fastest-growing region in the biomarker technologies market, projected to grow from $4.61 billion in 2023 to $10.00 billion by 2033.
Does ConsaInsights provide customized market report data for the biomarker Technologies industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the biomarker technologies industry, including detailed insights, trends, and market projections.
What deliverables can I expect from this biomarker Technologies market research project?
From this market research project, you can expect comprehensive reports detailing market analysis, trends, regional insights, and competitive landscapes specific to biomarker technologies.
What are the market trends of biomarker technologies?
Current trends in biomarker technologies include the rise of digital biomarkers, increased focus on oncology applications, and expanding uses in personalized medicine, reflecting a shift toward more targeted diagnostic solutions.