Biomarkers Market Report
Published Date: 31 January 2026 | Report Code: biomarkers
Biomarkers Market Size, Share, Industry Trends and Forecast to 2033
This market report provides a comprehensive overview of the Biomarkers industry, highlighting market size, growth trends, regional analyses, and future forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
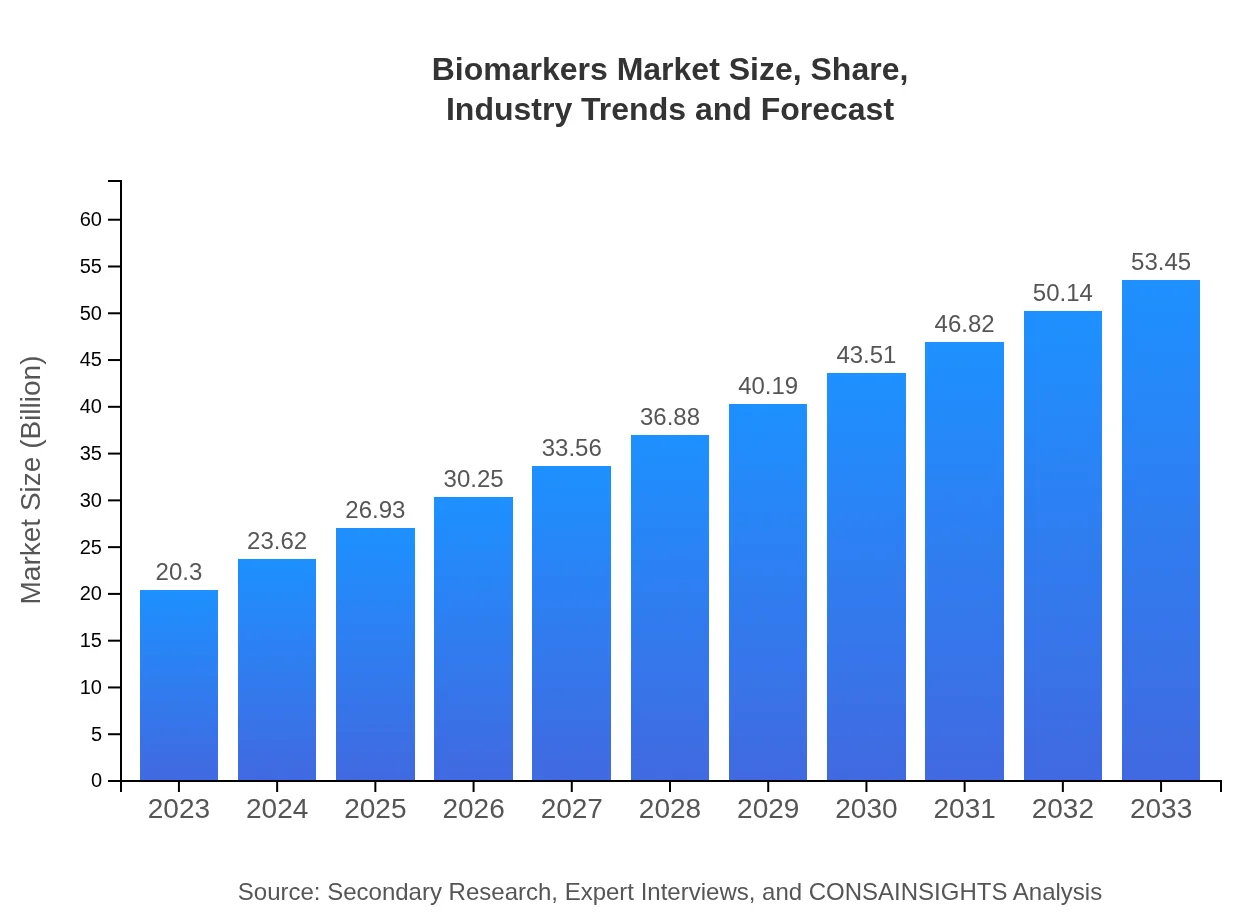
| 2023 Market Size | $20.30 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $53.45 Billion |
| Top Companies | Roche Diagnostics, Thermo Fisher Scientific, Abbott Laboratories, Quest Diagnostics, QIAGEN |
| Last Modified Date | 31 January 2026 |
Biomarkers Market Overview
Customize Biomarkers Market Report market research report
- ✔ Get in-depth analysis of Biomarkers market size, growth, and forecasts.
- ✔ Understand Biomarkers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biomarkers
What is the Market Size & CAGR of Biomarkers market in 2023?
Biomarkers Industry Analysis
Biomarkers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biomarkers Market Analysis Report by Region
Europe Biomarkers Market Report:
The European biomarkers market, estimated to grow from $5.12 billion in 2023 to $13.49 billion in 2033, benefits from high healthcare standards and regulatory support for biomarker research. The presence of prominent healthcare companies and increased investments in biotechnological innovations in countries like Germany, France, and the UK are expected to drive the market.Asia Pacific Biomarkers Market Report:
The Biomarkers market in Asia Pacific is expected to grow from $4.09 billion in 2023 to $10.77 billion by 2033. Factors driving this growth include increasing healthcare expenditures, rising chronic disease prevalence, and the adoption of advanced diagnostic technologies. Furthermore, government initiatives aimed at promoting biopharmaceuticals and personalized medicine are bolstering market expansion in countries like China and India.North America Biomarkers Market Report:
North America, specifically the United States, holds a dominant position in the biomarkers market, projected to expand from $7.59 billion in 2023 to $19.98 billion by 2033. Key factors include extensive investment in life sciences, robust regulatory frameworks, and a strong presence of leading biomarker technology companies. The region’s emphasis on personalized medicine and the rapid advancement of genomics and proteomics further catalyze market growth.South America Biomarkers Market Report:
The South American biomarkers market is projected to grow from $2.00 billion in 2023 to $5.25 billion by 2033. Growth will be influenced by improvements in healthcare infrastructure and increased investment in research and development. Countries such as Brazil are focusing on enhancing their diagnostic capabilities, reflecting in the growing adoption of advanced biomarker technologies in clinical practice.Middle East & Africa Biomarkers Market Report:
The Middle East and Africa biomarkers market is anticipated to grow from $1.51 billion in 2023 to $3.97 billion by 2033. Increasing awareness about chronic diseases and the gradual enhancement of healthcare infrastructure are expected to facilitate market growth, particularly in countries like the UAE and South Africa, where investments in healthcare technologies are rising.Tell us your focus area and get a customized research report.
Biomarkers Market Analysis By Type
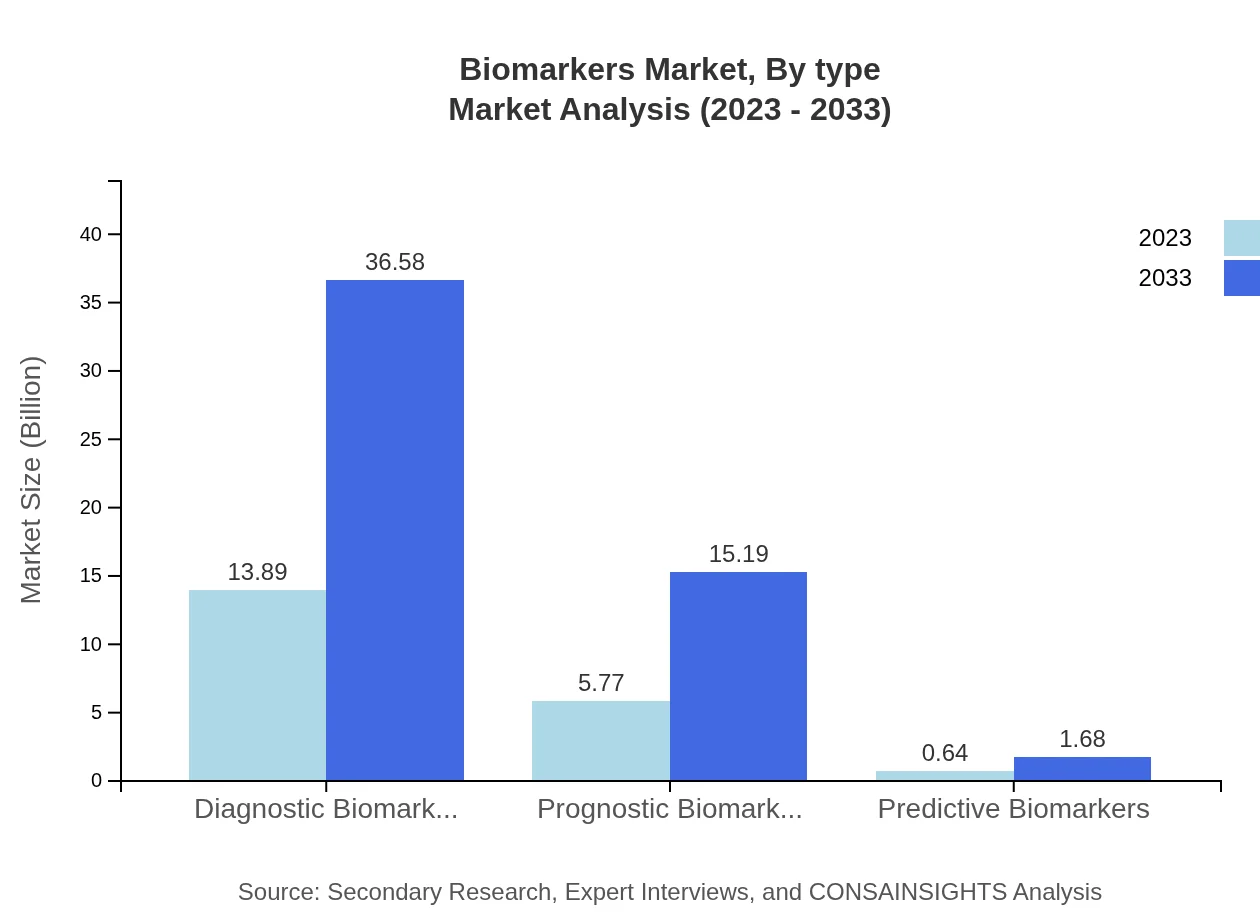
The biomarkers market is primarily segmented by type into diagnostic, prognostic, and predictive biomarkers. In 2023, diagnostic biomarkers lead the market with a size of $13.89 billion and are projected to increase to $36.58 billion by 2033, accounting for 68.43% market share. Prognostic biomarkers follow with a current market size of $5.77 billion, anticipating growth to $15.19 billion by 2033, representing 28.42% share. Predictive biomarkers, though smaller at $0.64 billion in 2023, are anticipated to reach $1.68 billion, making up 3.15% of the market.
Biomarkers Market Analysis By Technology
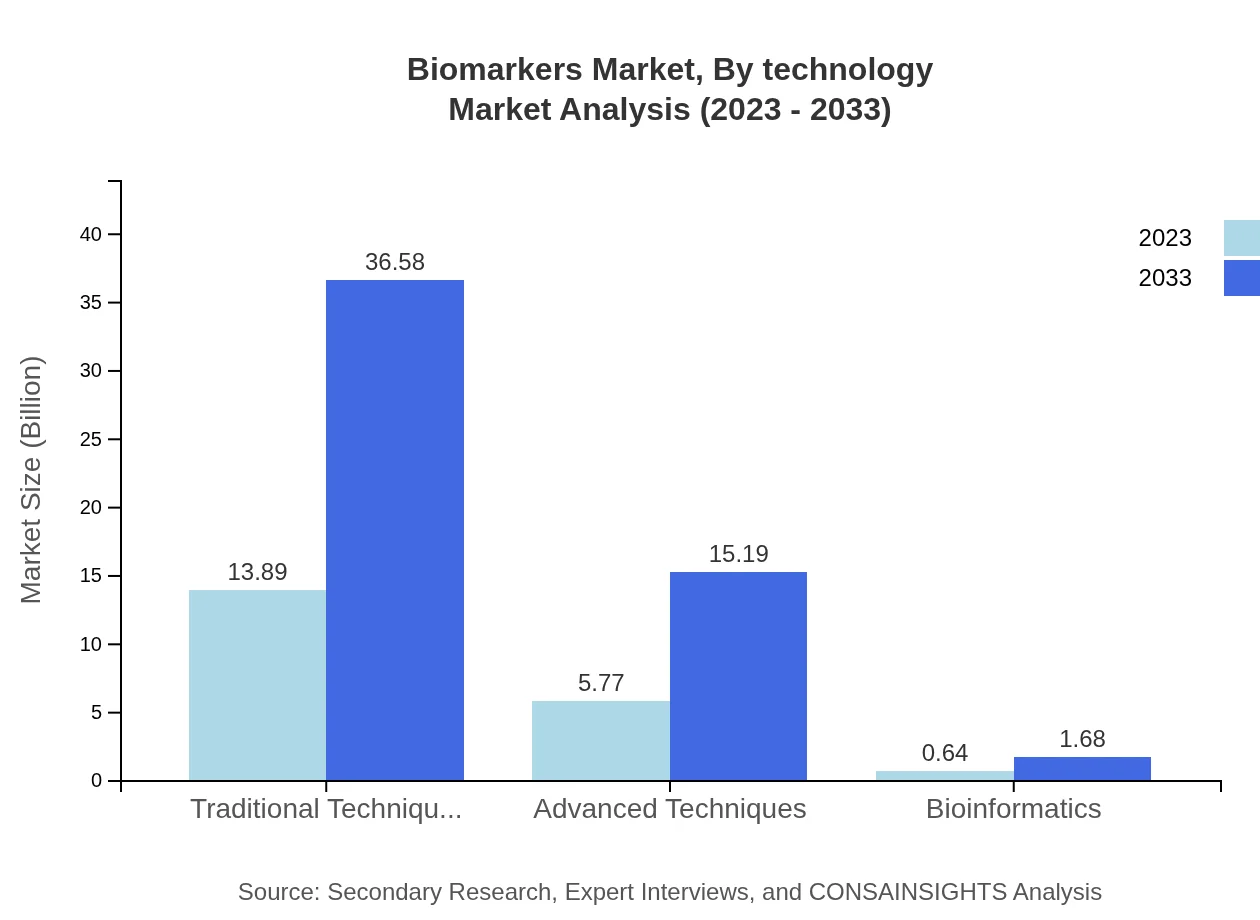
The technology segment of the biomarkers market is characterized by traditional and advanced techniques. Traditional techniques currently hold a significant share, size of $13.89 billion, and are expected to grow to $36.58 billion by 2033, maintaining a 68.43% share. Advanced techniques are also on the rise, from $5.77 billion in 2023 to an estimated $15.19 billion by 2033, equating to a 28.42% market share. Bioinformatics technologies are gaining traction, albeit at a smaller size of $0.64 billion in 2023, growing to $1.68 billion by 2033.
Biomarkers Market Analysis By Application
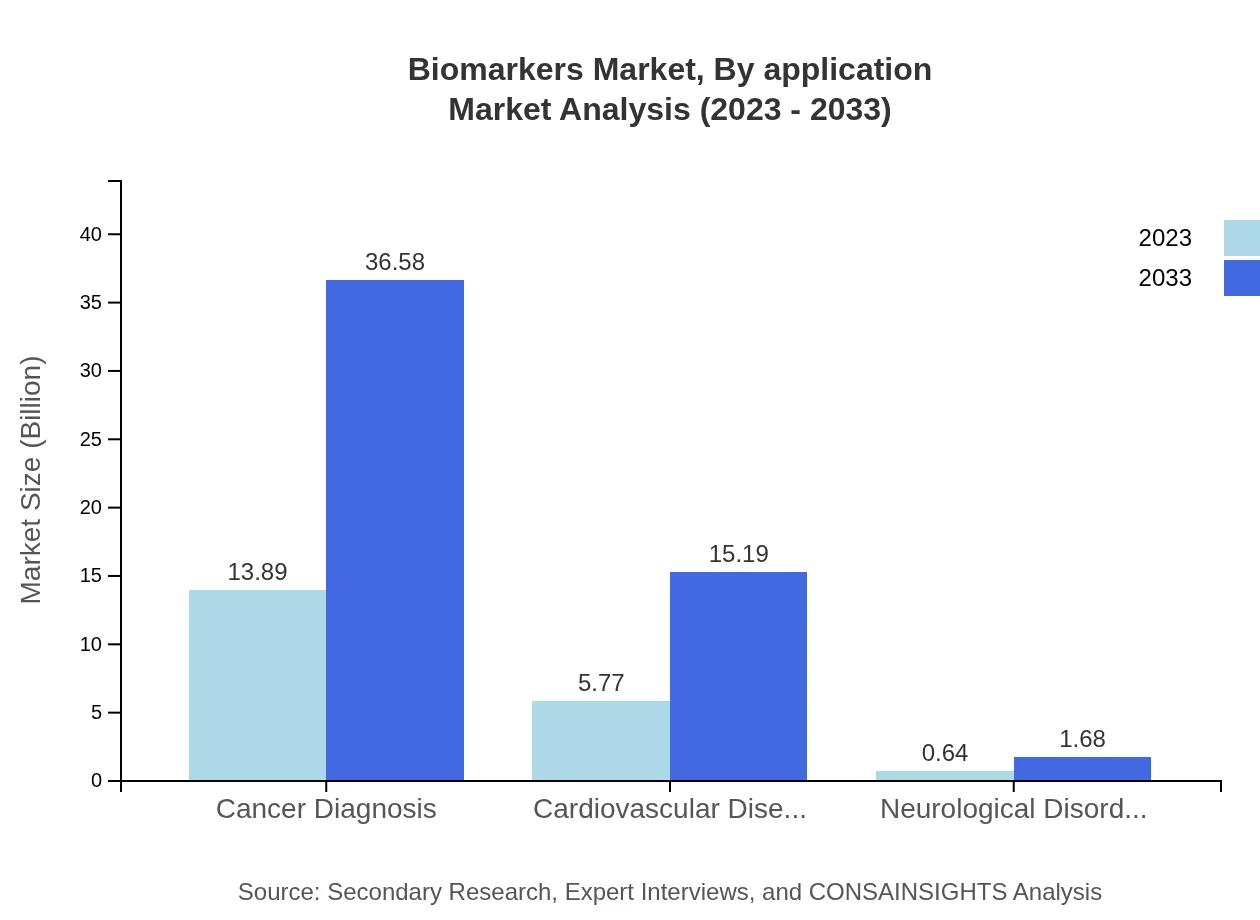
The application of biomarkers is predominantly in cancer diagnosis, cardiovascular diseases, and neurological disorders. The cancer diagnosis segment is currently the largest, valued at $13.89 billion in 2023 and expected to reach $36.58 billion by 2033, holding a market share of 68.43%. The cardiovascular diseases segment shows promise, with growth from $5.77 billion to $15.19 billion by 2033, which is 28.42% market share. Neurological disorders, while smaller with a size of $0.64 billion, are set to grow to $1.68 billion by 2033.
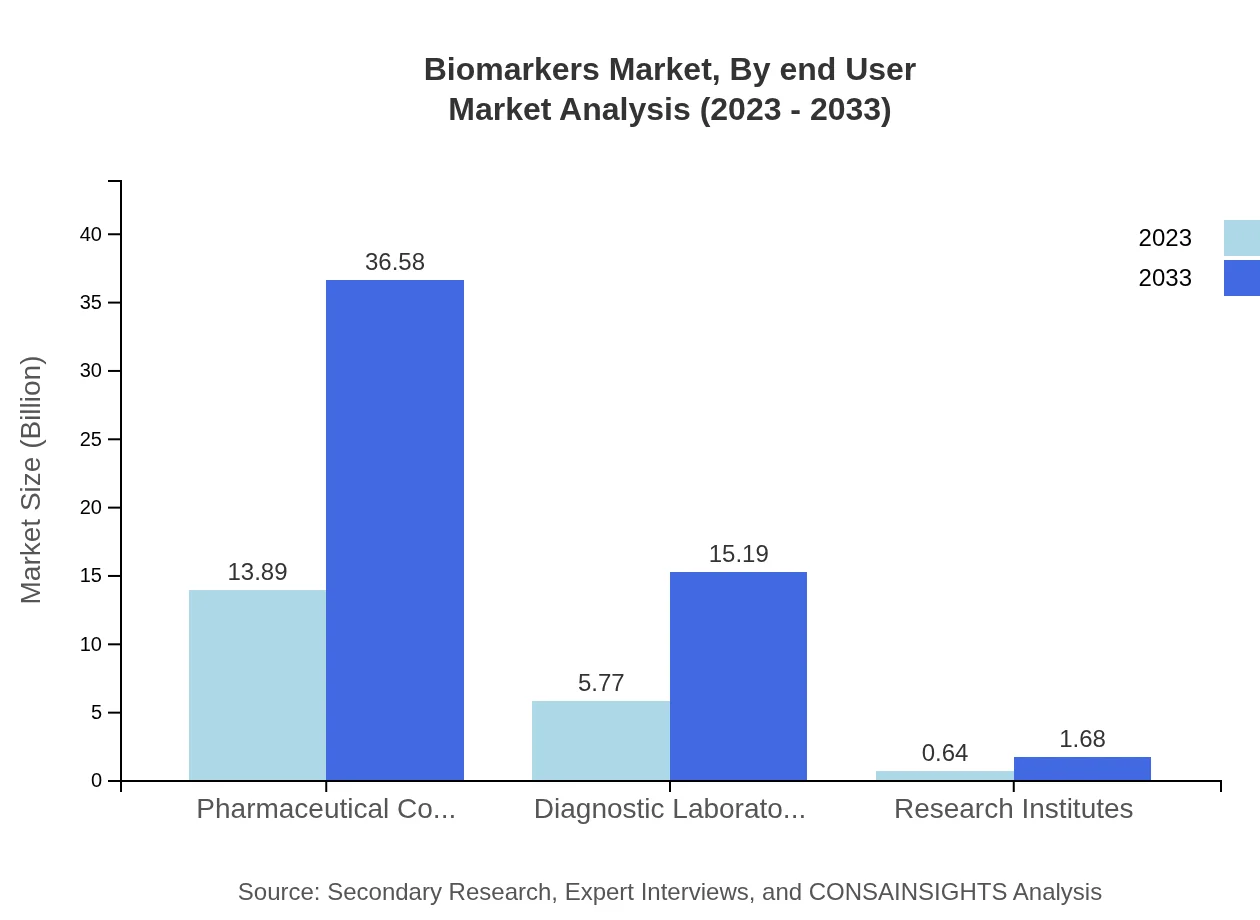
Biomarkers Market Analysis By End User
The end-user market for biomarkers consists of pharmaceutical companies, diagnostic laboratories, and research institutes. Pharmaceutical companies currently dominate the market with a size of $13.89 billion in 2023, projected to grow to $36.58 billion by 2033, capturing a 68.43% share. Diagnostic laboratories are expected to grow from $5.77 billion to $15.19 billion, reflecting 28.42% share, while research institutes, despite a smaller starting size of $0.64 billion, are set to grow to $1.68 billion.
Biomarkers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biomarkers Industry
Roche Diagnostics:
Roche Diagnostics is a leading company specializing in developing innovative testing solutions that leverage biomarkers for disease diagnosis. The company's focus on personalized medicine has made them a key player in the biomarker market.Thermo Fisher Scientific:
Thermo Fisher Scientific provides comprehensive solutions including advanced technologies in genomics and proteomics, enhancing biomarker discovery and application across multiple medical fields, reinforcing their position in the market.Abbott Laboratories:
Abbott Laboratories excels in diagnostics and pharmaceuticals, focusing on integrating biomarkers into their products, establishing their presence in both diagnostic and therapeutic areas.Quest Diagnostics:
Quest Diagnostics is a leading provider of diagnostic testing services, actively utilizing biomarkers to enhance diagnostic accuracy and patient management.QIAGEN :
QIAGEN offers pioneering technologies for biomarker discovery and validation using advanced molecular diagnostic techniques, further asserting their significance.We're grateful to work with incredible clients.









FAQs
What is the market size of biomarkers?
The biomarkers market was valued at approximately $20.3 billion in 2023, with a projected CAGR of 9.8%. By 2033, it's expected to significantly expand, reflecting the growing need for diagnostic and prognostic testing across various healthcare sectors.
What are the key market players or companies in this biomarkers industry?
Key players in the biomarkers market include pharmaceutical companies like Roche, Abbott Laboratories, and Thermo Fisher Scientific, who are leading the innovation in biomarker research and development, particularly in diagnostics and therapeutics.
What are the primary factors driving the growth in the biomarkers industry?
Growth in the biomarkers industry is driven by rising incidences of chronic diseases, advancements in technology, increased healthcare expenditure, and a strong focus on personalized medicine. Regulatory approvals also boost market opportunities for new biomarker-based diagnostics.
Which region is the fastest Growing in the biomarkers market?
The Asia Pacific region is the fastest-growing segment in the biomarkers market, with a market size expected to grow from $4.09 billion in 2023 to $10.77 billion by 2033, driven by improved healthcare infrastructure and increasing research activities.
Does ConsaInsights provide customized market report data for the biomarkers industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs in the biomarkers industry. This includes detailed insights, data analytics, and trend analyses to help businesses make informed decisions.
What deliverables can I expect from this biomarkers market research project?
Deliverables from the biomarkers market research include comprehensive market analysis, competitive landscape insights, growth forecasts, regional breakdowns, and detailed sector analyses, all tailored to client's business objectives.
What are the market trends of biomarkers?
Current trends in the biomarkers market include increasing demand for companion diagnostics, growth in genomics and personalized medicine, heightened focus on early disease detection, and technological advancements in AI and bioinformatics enhancing biomarker discovery.