Biomedical Pressure Sensors Market Report
Published Date: 31 January 2026 | Report Code: biomedical-pressure-sensors
Biomedical Pressure Sensors Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive overview of the Biomedical Pressure Sensors market, analyzing current trends, market size, and growth projections from 2023 to 2033. Insights include regional breakdowns and detailed segment analyses to guide stakeholders in strategic decision-making.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
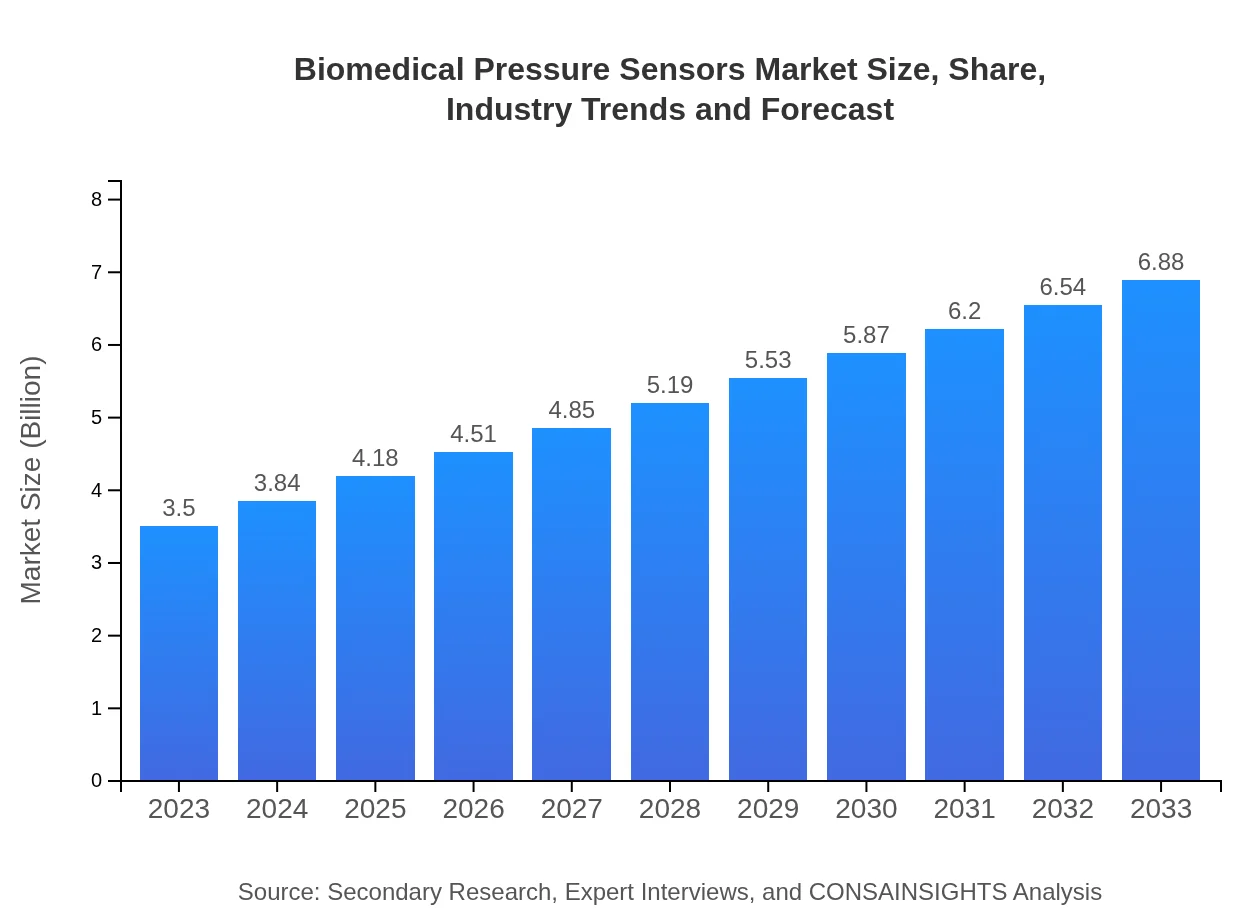
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $6.88 Billion |
| Top Companies | Honeywell International Inc., Bosch Sensortec, Medtronic , ABB Ltd. |
| Last Modified Date | 31 January 2026 |
Biomedical Pressure Sensors Market Overview
Customize Biomedical Pressure Sensors Market Report market research report
- ✔ Get in-depth analysis of Biomedical Pressure Sensors market size, growth, and forecasts.
- ✔ Understand Biomedical Pressure Sensors's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biomedical Pressure Sensors
What is the Market Size & CAGR of Biomedical Pressure Sensors Market in 2023?
Biomedical Pressure Sensors Industry Analysis
Biomedical Pressure Sensors Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biomedical Pressure Sensors Market Analysis Report by Region
Europe Biomedical Pressure Sensors Market Report:
The European market is set to grow from $0.91 billion in 2023 to $1.80 billion by 2033, driven by stringent regulatory requirements and increasing demand for advanced medical devices across both public and private healthcare sectors.Asia Pacific Biomedical Pressure Sensors Market Report:
In the Asia Pacific region, the market for Biomedical Pressure Sensors is projected to grow from $0.69 billion in 2023 to $1.35 billion by 2033, driven by rapid advancements in healthcare technology and increased healthcare spending in countries like China and India.North America Biomedical Pressure Sensors Market Report:
North America remains the largest market, with a projected increase from $1.35 billion in 2023 to $2.66 billion by 2033. The region benefits from high adoption rates of innovative technologies and a strong emphasis on patient monitoring systems.South America Biomedical Pressure Sensors Market Report:
The South American market is expected to grow from $0.29 billion in 2023 to $0.58 billion by 2033. Growth factors include an expanding healthcare infrastructure and increased accessibility to advanced medical devices.Middle East & Africa Biomedical Pressure Sensors Market Report:
The Middle East and Africa are anticipated to witness market growth from $0.25 billion in 2023 to $0.50 billion by 2033, fueled by rising healthcare investments and the expansion of medical device markets in emerging economies.Tell us your focus area and get a customized research report.
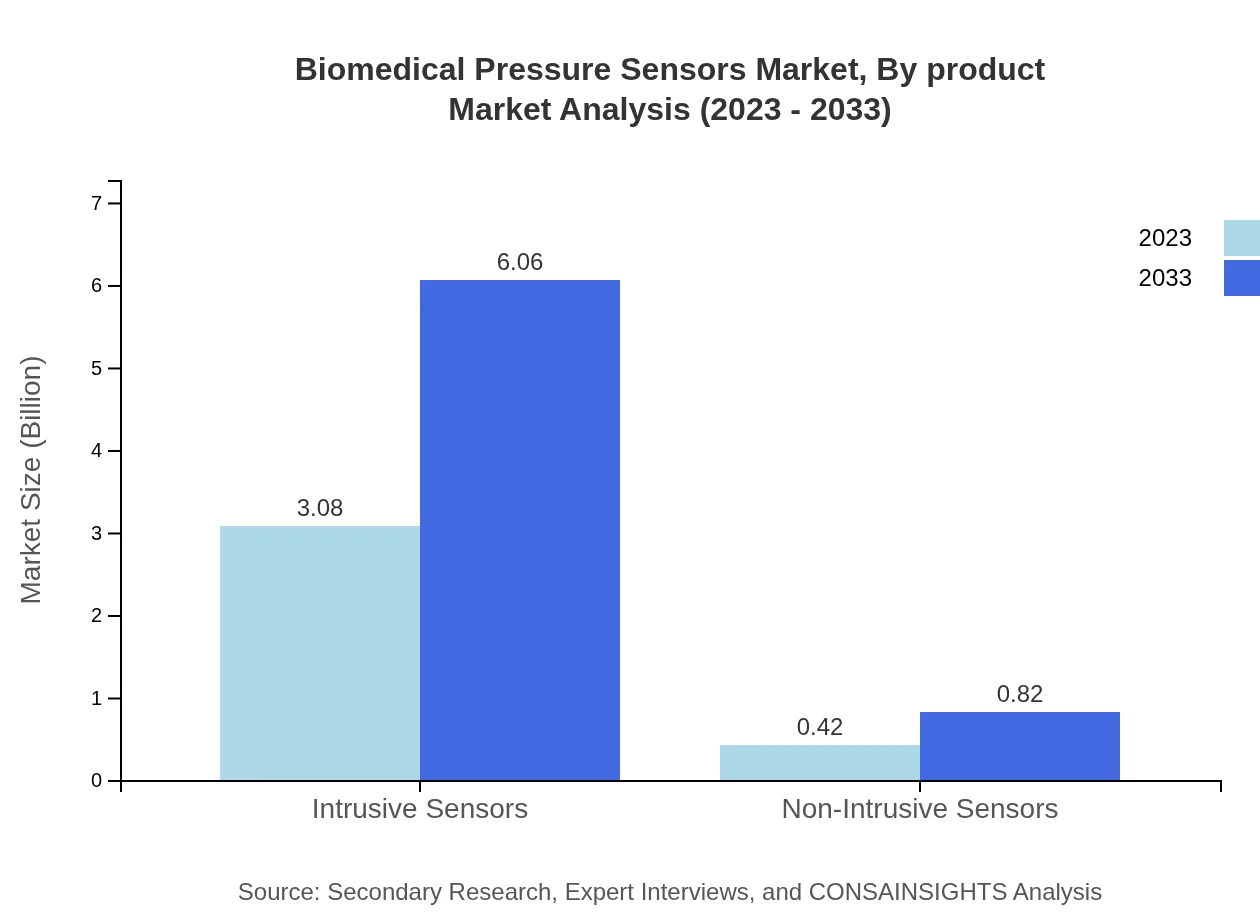
Biomedical Pressure Sensors Market Analysis By Product
The product segmentation of Biomedical Pressure Sensors is primarily divided into Intrusive and Non-Intrusive sensors. Intrusive Sensors are estimated to have a market size of $3.08 billion by 2033, holding 88.12% market share, while Non-Intrusive Sensors are expected to grow to $0.82 billion, representing an 11.88% share.
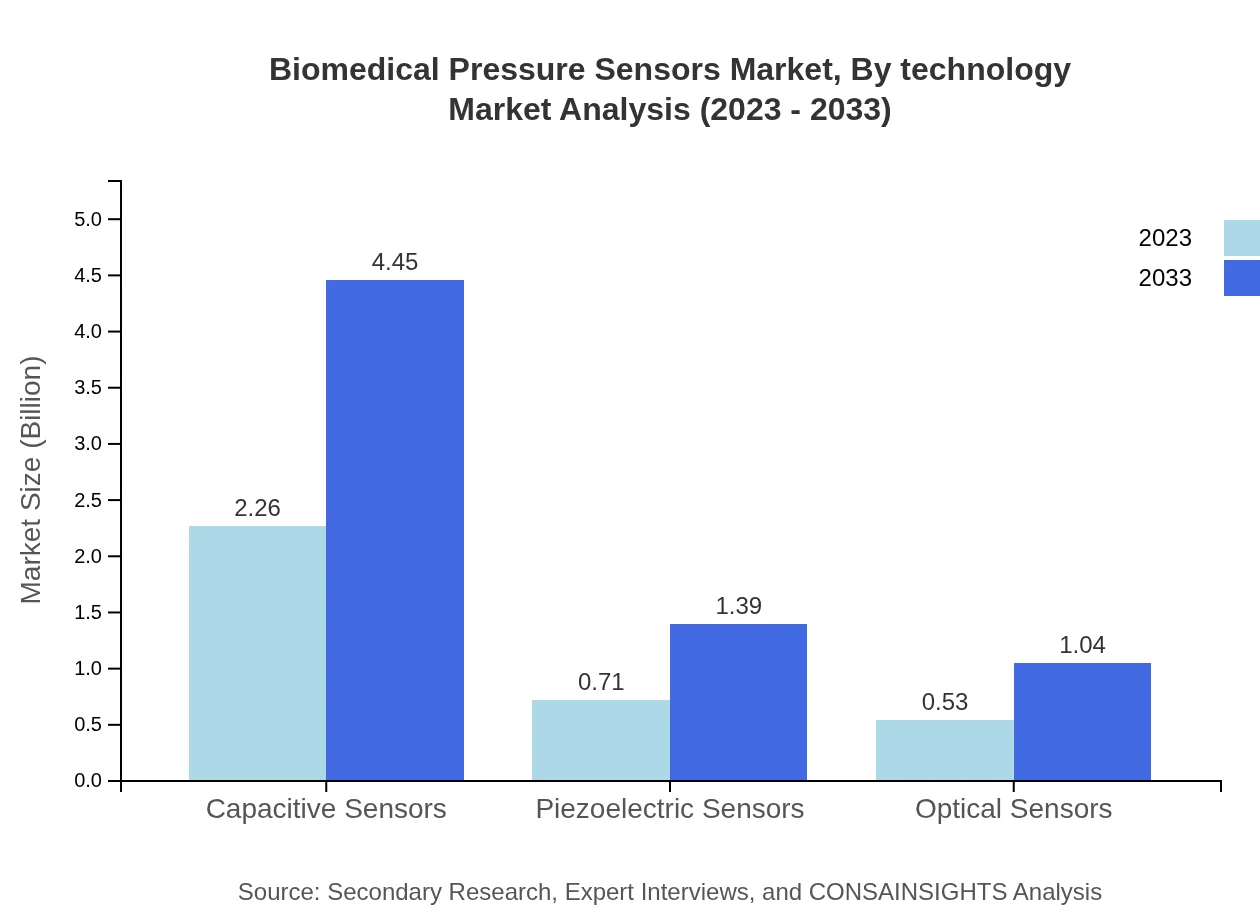
Biomedical Pressure Sensors Market Analysis By Technology
By technology, Capacitive Sensors dominate the market, projected at $4.45 billion or 64.68% by 2033, followed by Piezoelectric Sensors at $1.39 billion (20.27%) and Optical Sensors at $1.04 billion (15.05%). This segmentation reflects the technological advancements and applications of sensor types across various medical fields.
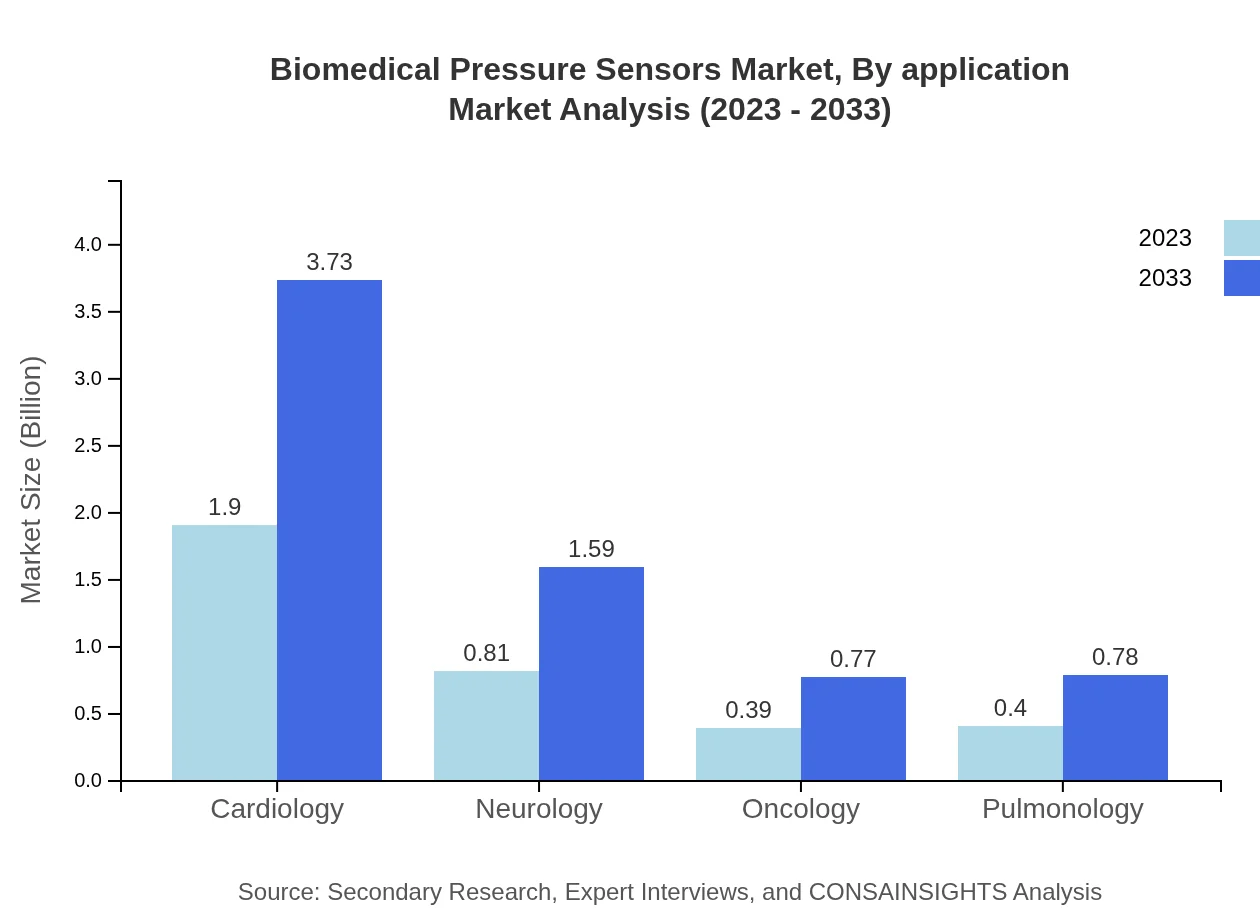
Biomedical Pressure Sensors Market Analysis By Application
The application of Biomedical Pressure Sensors spans various medical specialties, with Cardiology leading the market with $3.73 billion (54.26%) by 2033. Other significant applications include Neurology at $1.59 billion (23.18%), Oncology at $0.77 billion (11.24%), and Pulmonology at $0.78 billion (11.32%), underscoring the diverse applicability of these sensors in critical healthcare.
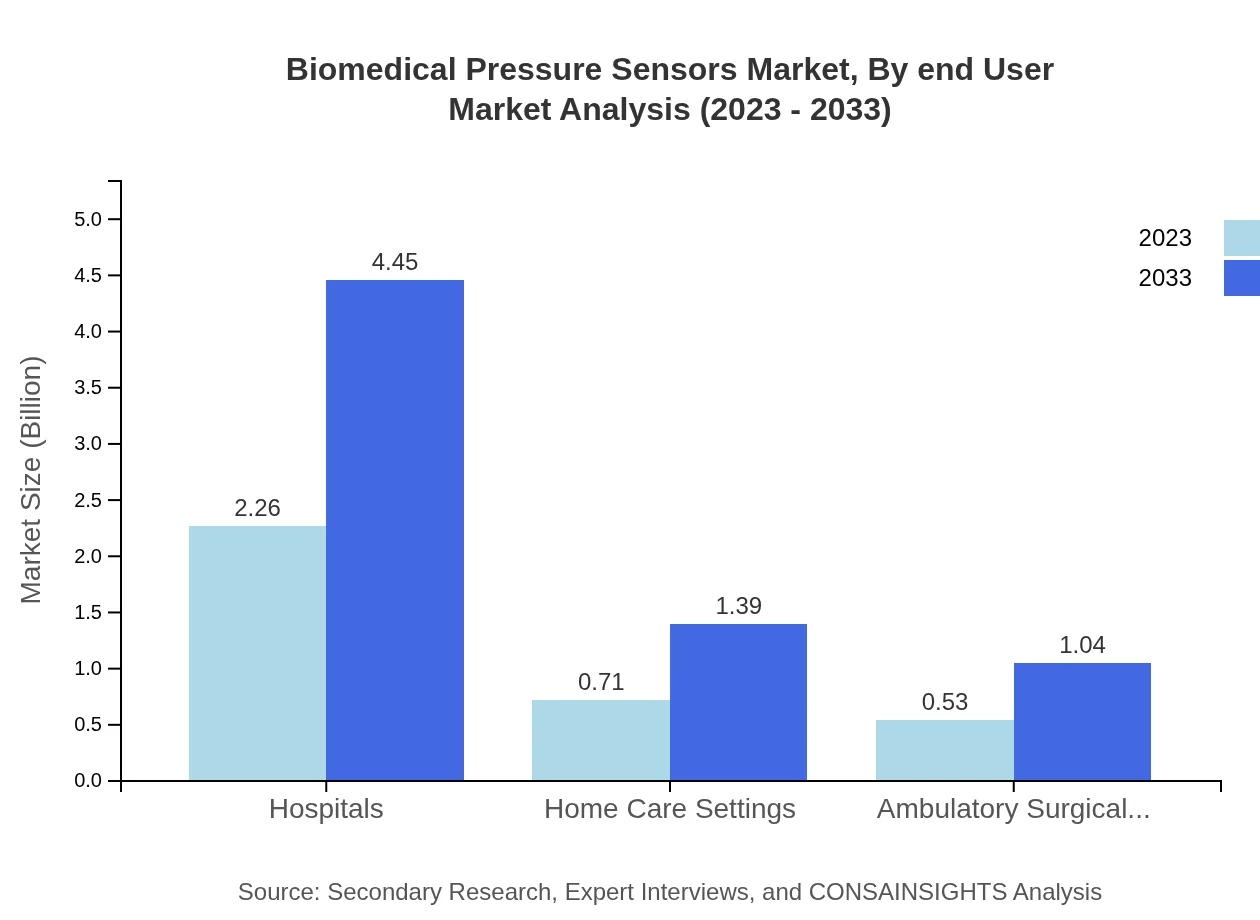
Biomedical Pressure Sensors Market Analysis By End User
End-user analysis shows Hospitals commanding a major share at $4.45 billion (64.68%) by 2033, followed by Home Care Settings at $1.39 billion (20.27%) and Ambulatory Surgical Centers at $1.04 billion (15.05%). This reflects the growing trend toward patient-centered care and the shift towards home healthcare solutions.
Biomedical Pressure Sensors Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biomedical Pressure Sensors Industry
Honeywell International Inc.:
A global leader in sensor technologies, Honeywell offers advanced biomedical pressure sensors for various applications, significantly contributing to patient monitoring systems.Bosch Sensortec:
Bosch Sensortec specializes in micro-electromechanical systems (MEMS) and biomedical sensors, providing innovative solutions for medical devices.Medtronic :
Medtronic, a major player in healthcare technology, develops integrated pressure sensing technologies that enhance patient diagnostics and monitoring.ABB Ltd.:
ABB is known for its expertise in automation and smart sensors, providing critical solutions that improve precision in biomedical applications.We're grateful to work with incredible clients.









FAQs
What is the market size of biomedical Pressure Sensors?
The biomedical pressure sensors market is projected to reach a size of approximately $3.5 billion in 2023, with an expected CAGR of 6.8% over the next decade. This growth indicates increasing demand for accurate monitoring devices in healthcare.
What are the key market players or companies in this biomedical Pressure Sensors industry?
The leading companies in the biomedical pressure sensors market include renowned organizations such as Siemens Healthineers, Honeywell, and Medtronic. These companies are pivotal in driving innovation and technology in the sector.
What are the primary factors driving the growth in the biomedical Pressure Sensors industry?
Key drivers for growth in the biomedical pressure sensors industry include advancements in healthcare technology, an aging population requiring consistent monitoring, and an increasing prevalence of chronic diseases necessitating precise pressure measurements.
Which region is the fastest Growing in the biomedical Pressure Sensors?
The Asia Pacific region is emerging as the fastest-growing area for biomedical pressure sensors, expected to grow from $0.69 billion in 2023 to $1.35 billion by 2033, driven by increasing healthcare expenditure and technological adoption.
Does ConsaInsights provide customized market report data for the biomedical Pressure Sensors industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the biomedical pressure sensors industry, allowing businesses to access unique insights relevant to their strategic goals.
What deliverables can I expect from this biomedical Pressure Sensors market research project?
Deliverables from the biomedical pressure sensors market research project typically include comprehensive market analysis, segmentation data, forecasts, competitive landscape overviews, and strategic recommendations based on the findings.
What are the market trends of biomedical Pressure Sensors?
Current market trends in the biomedical pressure sensors field focus on innovations in sensor technologies, increased integration of IoT devices for real-time monitoring, and a growing preference for non-invasive measurement methods to enhance patient comfort.