Biosimilar Monoclonal Antibody Market Report
Published Date: 31 January 2026 | Report Code: biosimilar-monoclonal-antibody
Biosimilar Monoclonal Antibody Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the biosimilar monoclonal antibody market, covering key insights, market trends, and forecasts from 2023 to 2033. It highlights various segments, regional dynamics, and future growth potential within this vital healthcare sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
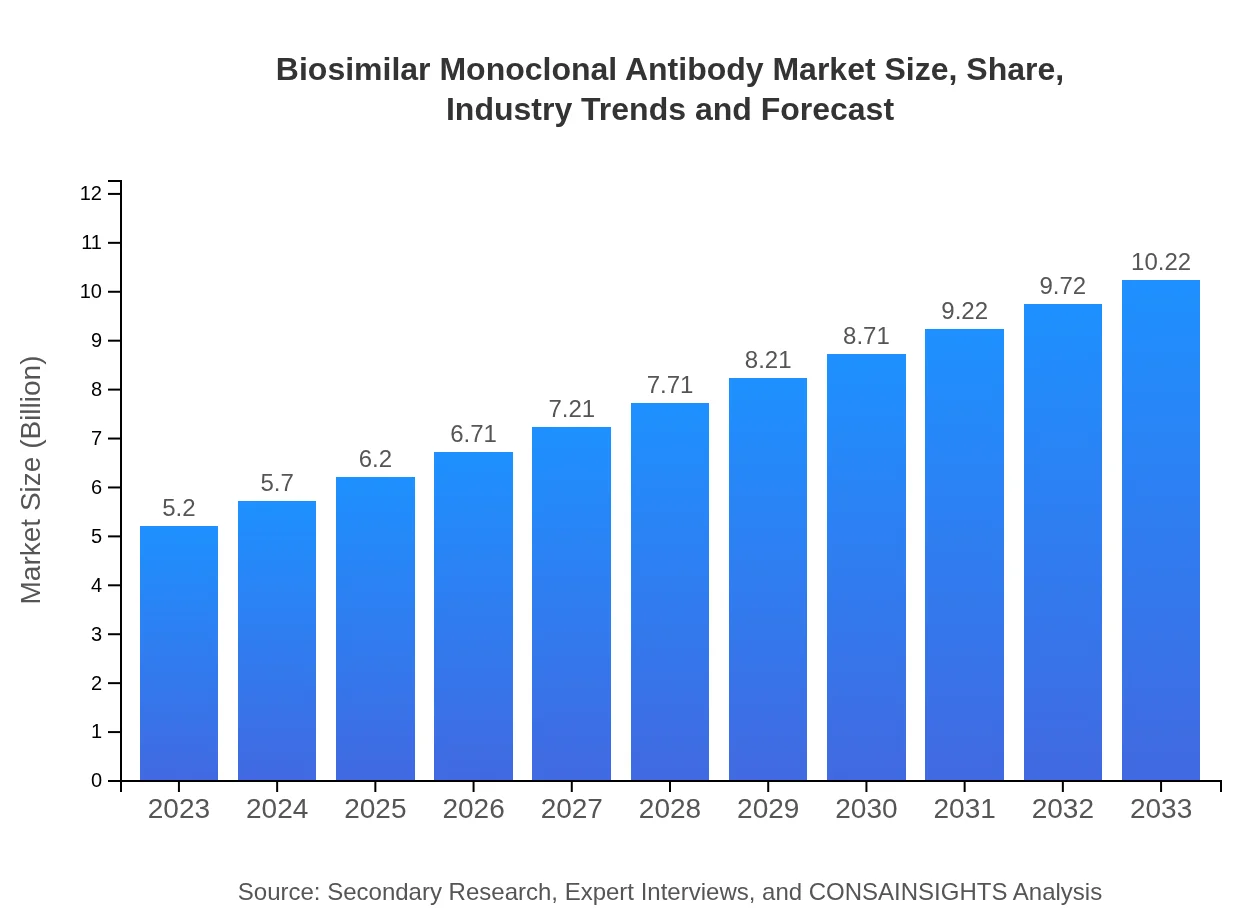
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Amgen Inc., Sandoz (a Novartis division), Pfizer Inc., Samsung Bioepis, AbbVie Inc. |
| Last Modified Date | 31 January 2026 |
Biosimilar Monoclonal Antibody Market Overview
Customize Biosimilar Monoclonal Antibody Market Report market research report
- ✔ Get in-depth analysis of Biosimilar Monoclonal Antibody market size, growth, and forecasts.
- ✔ Understand Biosimilar Monoclonal Antibody's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biosimilar Monoclonal Antibody
What is the Market Size & CAGR of the Biosimilar Monoclonal Antibody market in 2023?
Biosimilar Monoclonal Antibody Industry Analysis
Biosimilar Monoclonal Antibody Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Biosimilar Monoclonal Antibody Market Analysis Report by Region
Europe Biosimilar Monoclonal Antibody Market Report:
Europe's biosimilar monoclonal antibody market is expected to grow from $1.37 billion in 2023 to $2.70 billion by 2033. The region benefits from early adopters of biosimilar drugs and stringent policies promoting affordable healthcare solutions.Asia Pacific Biosimilar Monoclonal Antibody Market Report:
In the Asia Pacific region, the biosimilar monoclonal antibody market is projected to grow from $1.03 billion in 2023 to $2.03 billion by 2033. Factors driving this growth include increasing healthcare infrastructure, rising prevalence of chronic diseases, and growing investments in biopharmaceutical developments.North America Biosimilar Monoclonal Antibody Market Report:
North America dominates the biosimilar monoclonal antibody market, with a market size of $1.93 billion in 2023 projected to reach $3.80 billion by 2033. The presence of established healthcare systems, ongoing clinical trials, and strong regulatory support for biosimilars contribute to its leading position.South America Biosimilar Monoclonal Antibody Market Report:
The South American market reflects significant potential, with an increase from $0.16 billion to $0.31 billion from 2023 to 2033. The focus on improving access to treatments and the rising prevalence of health issues are key growth facilitators in this region.Middle East & Africa Biosimilar Monoclonal Antibody Market Report:
In the Middle East and Africa, the market size is estimated to rise from $0.70 billion to $1.37 billion from 2023 to 2033. Increased focus on healthcare reforms and rising awareness of biosimilars' benefits are expected to drive this growth.Tell us your focus area and get a customized research report.
Biosimilar Monoclonal Antibody Market Analysis By Product Class
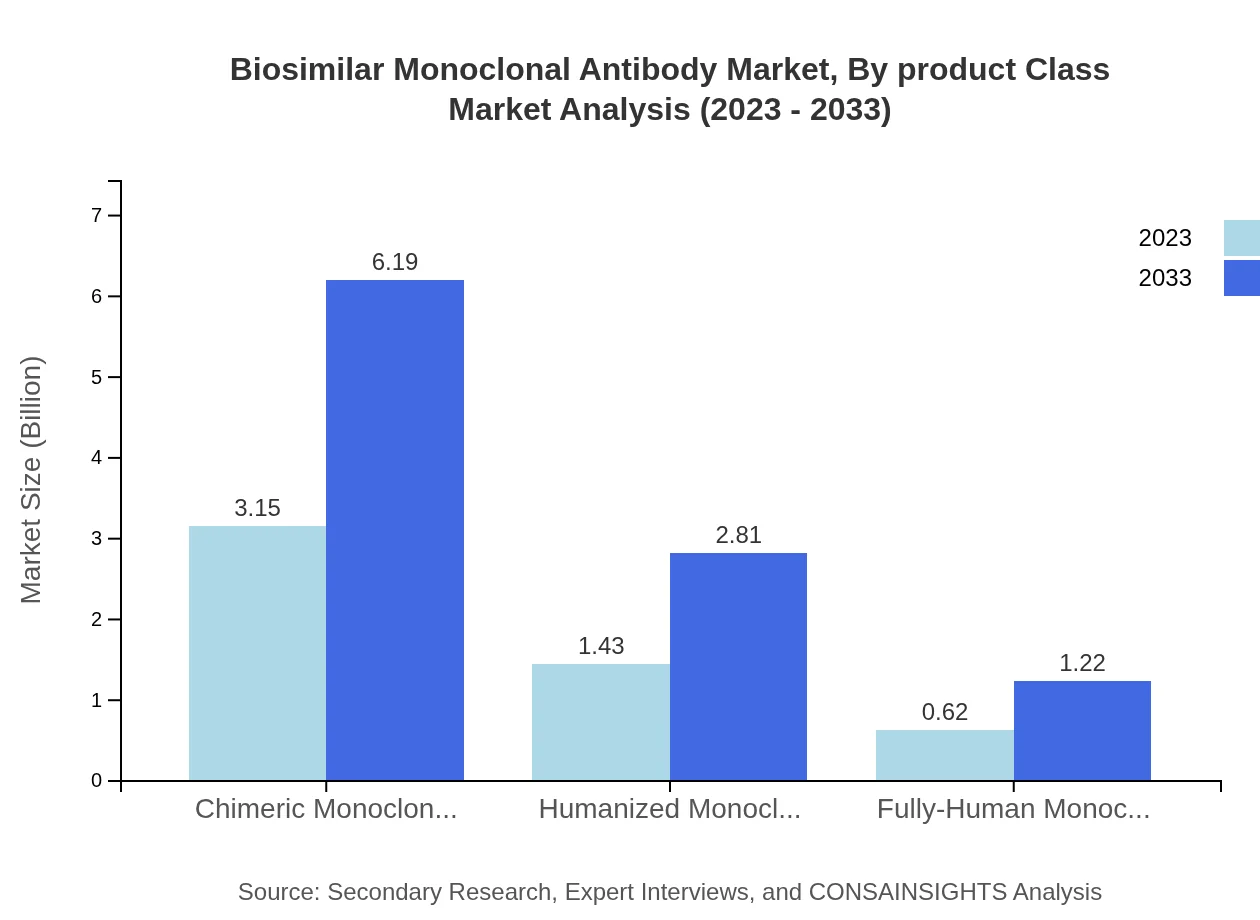
The market is categorized into chimeric, humanized, and fully-human monoclonal antibodies. Chimeric antibodies hold a significant market share of approximately 60.53% in 2023, expected to maintain the same through 2033. Humanized and fully-human monoclonal antibodies account for 27.51% and 11.96% market shares, respectively, presenting unique advantages in targeting specificity and reducing immunogenicity, contributing to their growing acceptance.
Biosimilar Monoclonal Antibody Market Analysis By Therapeutic Area
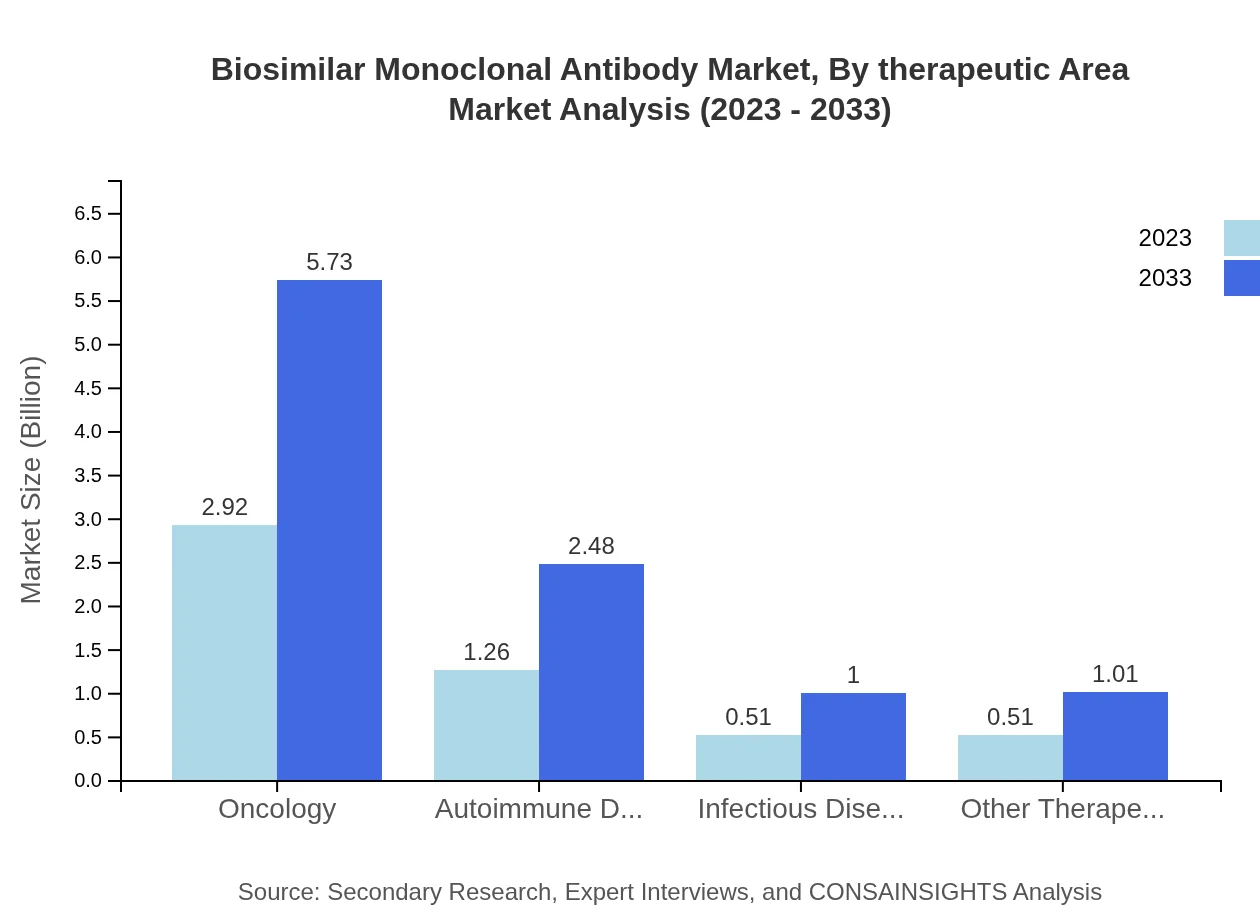
In therapeutic area segmentation, oncology leads with a significant market size of $2.92 billion in 2023, projected to increase to $5.73 billion by 2033. Autoimmune disorders follow with an increase from $1.26 billion to $2.48 billion. Infectious diseases and other therapeutic areas are also vital, representing niches that are expected to expand rapidly due to increasing healthcare demands.
Biosimilar Monoclonal Antibody Market Analysis By Manufacturing Process
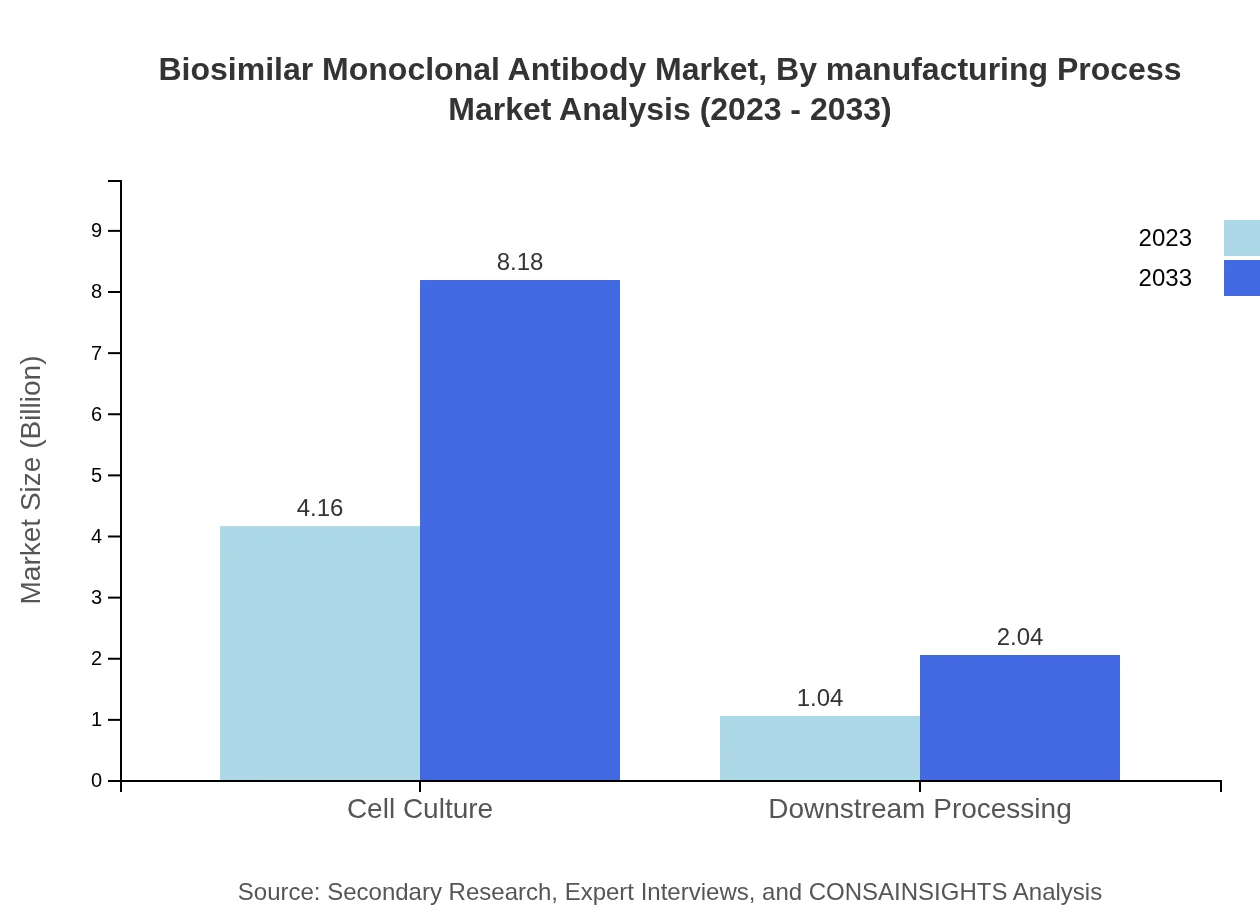
Regarding manufacturing processes, cell culture dominates with a market size of $4.16 billion in 2023, representing 80.05% share, which is crucial to scaling production capabilities effectively. Downstream processing, forming a smaller segment at 19.95%, is also anticipated to grow due to innovative purification techniques enhancing product yield and quality.
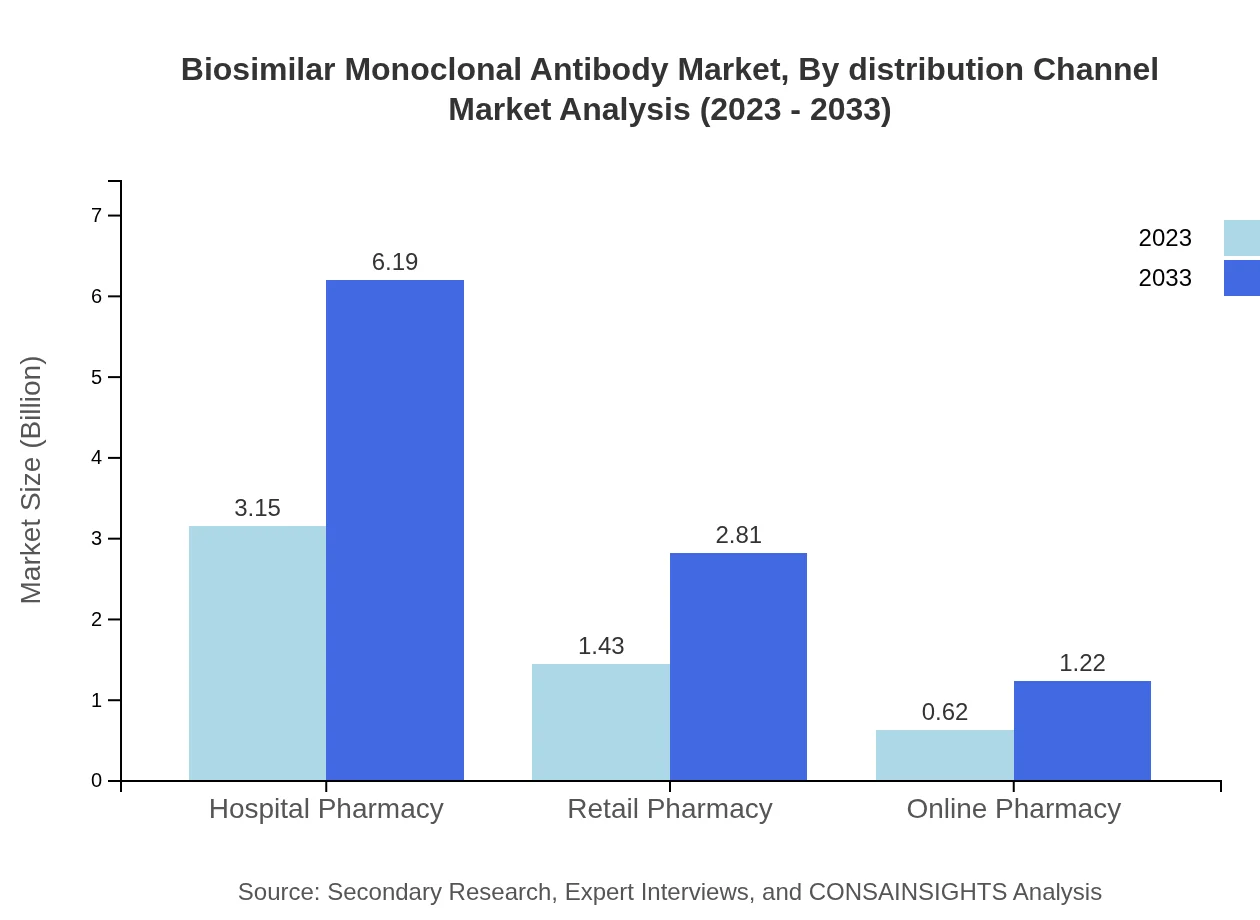
Biosimilar Monoclonal Antibody Market Analysis By Distribution Channel
Distribution channels include hospital pharmacies, retail pharmacies, and online platforms. Hospital pharmacies are predominant, holding a 60.53% share in 2023, while retail pharmacies and online channels constitute 27.51% and 11.96%, respectively. The convenience and accessibility of online pharmacies are pushing growth in this channel, especially in regions with evolving digital health ecosystems.
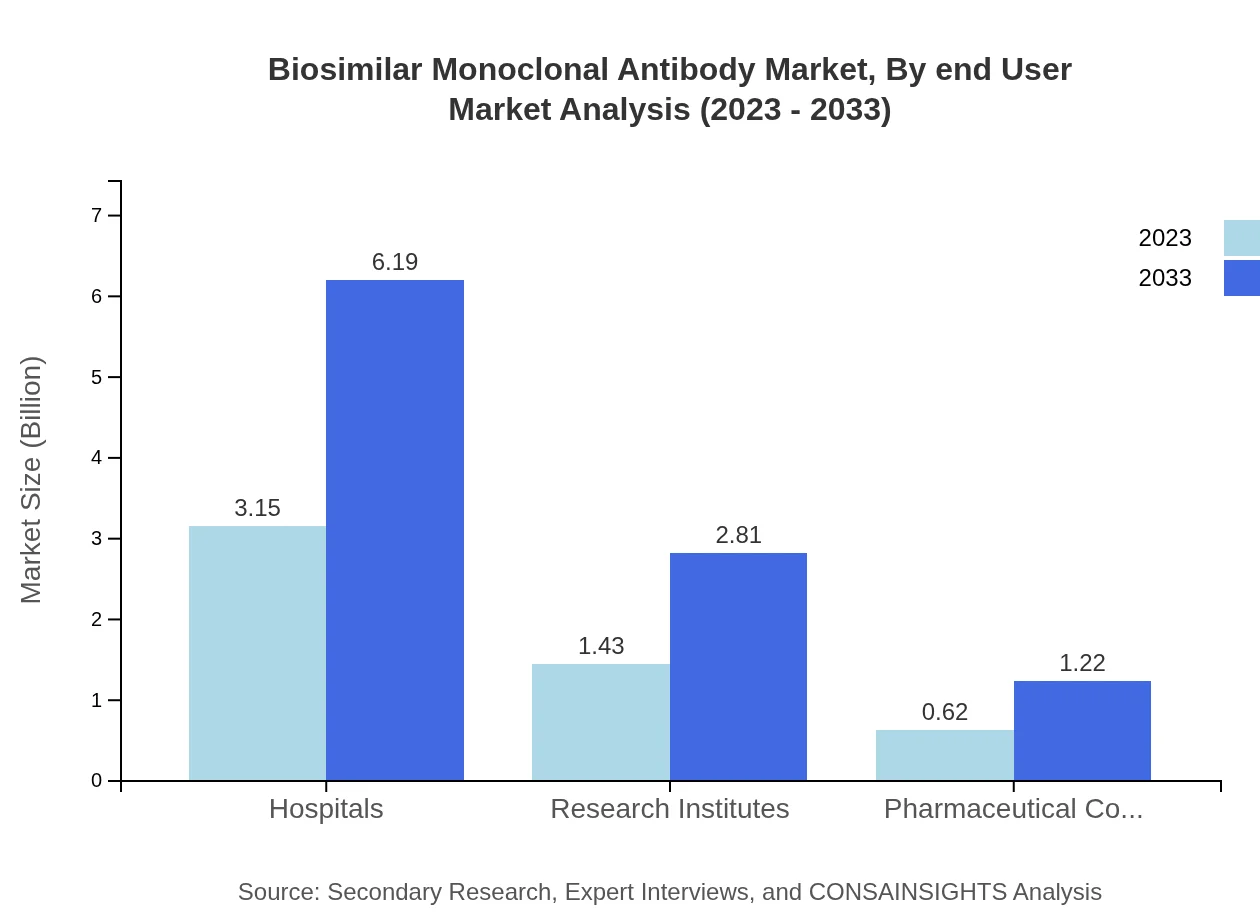
Biosimilar Monoclonal Antibody Market Analysis By End User
End-user segmentation reveals that hospitals are the largest market segment, accounting for a significant portion of the sales. Research institutes and pharmaceutical companies follow, representing critical stakeholders in developing and deploying biosimilar therapies. Their integrated roles in treatment and research initiatives are essential for sustaining market growth and innovation.
Biosimilar Monoclonal Antibody Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biosimilar Monoclonal Antibody Industry
Amgen Inc.:
Amgen is a pioneer in biotechnology with a strong portfolio of biosimilars, focusing on advancing patient access to high-quality therapies.Sandoz (a Novartis division):
Sandoz leads in the biosimilars space, offering a diverse range of products and demonstrating a commitment to sustainability in biopharmaceutical manufacturing.Pfizer Inc.:
Pfizer has developed several biosimilars that provide cost-effective treatment options, expanding their global market footprint considerably.Samsung Bioepis:
Samsung Bioepis specializes in innovative biopharmaceuticals, with an emphasis on biosimilars that drive patient accessibility and treatment effectiveness.AbbVie Inc.:
AbbVie invests heavily in biopharmaceutical research, including biosimilars, with a focus on improving therapeutic outcomes and patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of biosimilar Monoclonal Antibody?
The biosimilar monoclonal antibody market is projected to reach approximately $5.2 billion by 2033, growing at a CAGR of 6.8% from current levels. This growth will be driven by increasing demand and advancements in technology.
What are the key market players or companies in this biosimilar Monoclonal Antibody industry?
Key players in the biosimilar monoclonal antibody market include large pharmaceutical firms such as Amgen, Pfizer, and Biocon, which are all competing to innovate and capture market share of this rapidly expanding sector.
What are the primary factors driving the growth in the biosimilar Monoclonal Antibody industry?
Primary factors driving growth include the rising prevalence of chronic diseases, increasing healthcare costs, and the need for more affordable treatment options, which biosimilars provide relative to branded monoclonal antibodies.
Which region is the fastest Growing in the biosimilar Monoclonal Antibody?
The Asia Pacific region is poised to be the fastest-growing market for biosimilar monoclonal antibodies, with an expected increase from $1.03 billion in 2023 to $2.03 billion by 2033, reflecting widespread adoption and regulatory support.
Does ConsaInsights provide customized market report data for the biosimilar Monoclonal Antibody industry?
Yes, ConsaInsights offers customized market report data tailored to specific research needs in the biosimilar monoclonal antibody industry, providing insights that align with unique business objectives and market questions.
What deliverables can I expect from this biosimilar Monoclonal Antibody market research project?
Deliverables include comprehensive market analysis reports, regional data breakdowns, competitive landscape assessments, and segment analysis, ensuring stakeholders have a thorough understanding of market dynamics.
What are the market trends of biosimilar Monoclonal Antibody?
Current trends in the biosimilar monoclonal antibody market include increasing regulatory approvals, growing partnerships between companies, and a shift towards biologics in treatment protocols, leading to greater market accessibility.