Biosimulation Market Report
Published Date: 31 January 2026 | Report Code: biosimulation
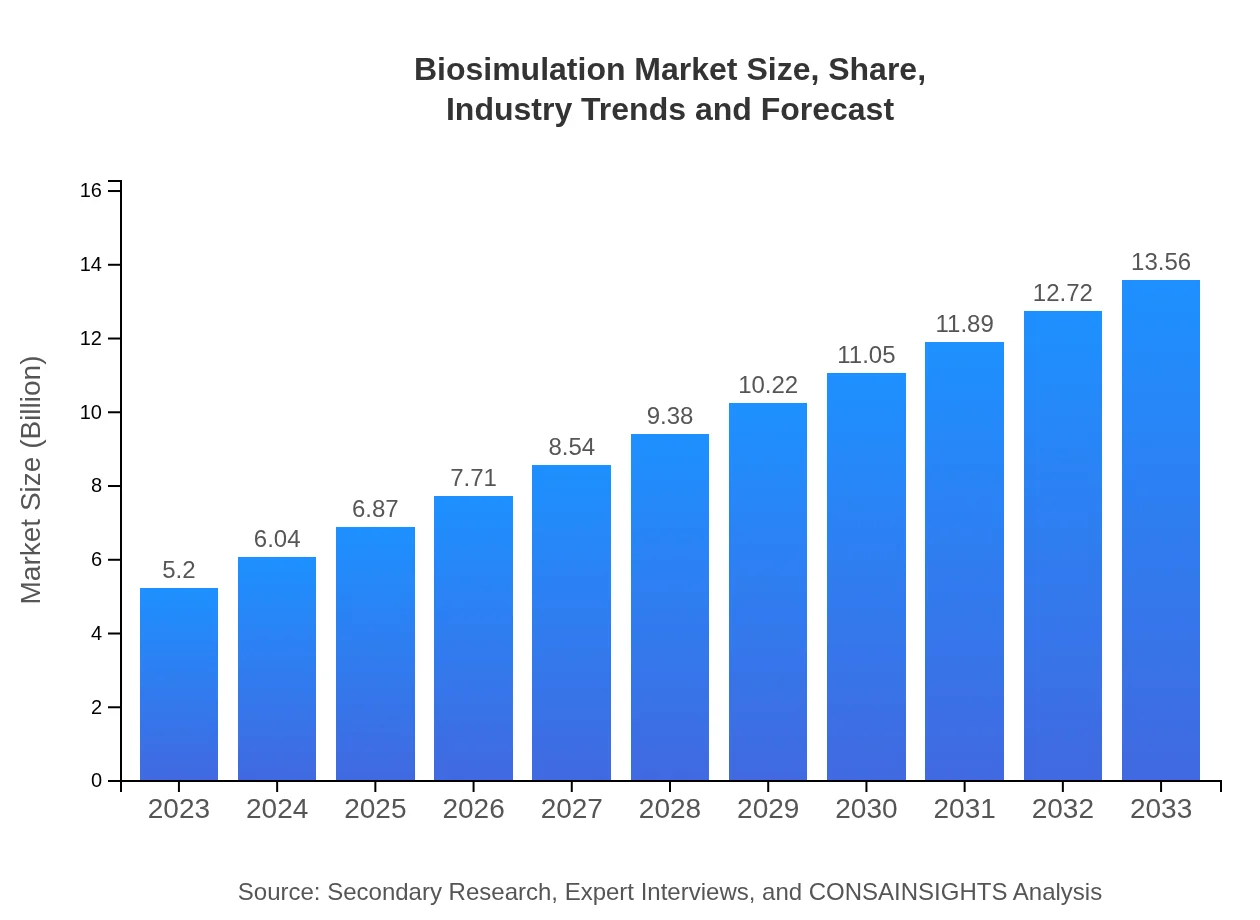
Biosimulation Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the biosimulation market from 2023 to 2033, highlighting key trends, current market conditions, segmentation, regional insights, and a forecast of growth and challenges in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 9.7% |
| 2033 Market Size | $13.56 Billion |
| Top Companies | Certara, Simulations Plus, Rhenovia Pharma, Insilico Medicine |
| Last Modified Date | 31 January 2026 |
Biosimulation Market Overview
Customize Biosimulation Market Report market research report
- ✔ Get in-depth analysis of Biosimulation market size, growth, and forecasts.
- ✔ Understand Biosimulation's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Biosimulation
What is the Market Size & CAGR of Biosimulation market in 2023?
Biosimulation Industry Analysis
Biosimulation Market Segmentation and Scope
Tell us your focus area and get a customized research report.
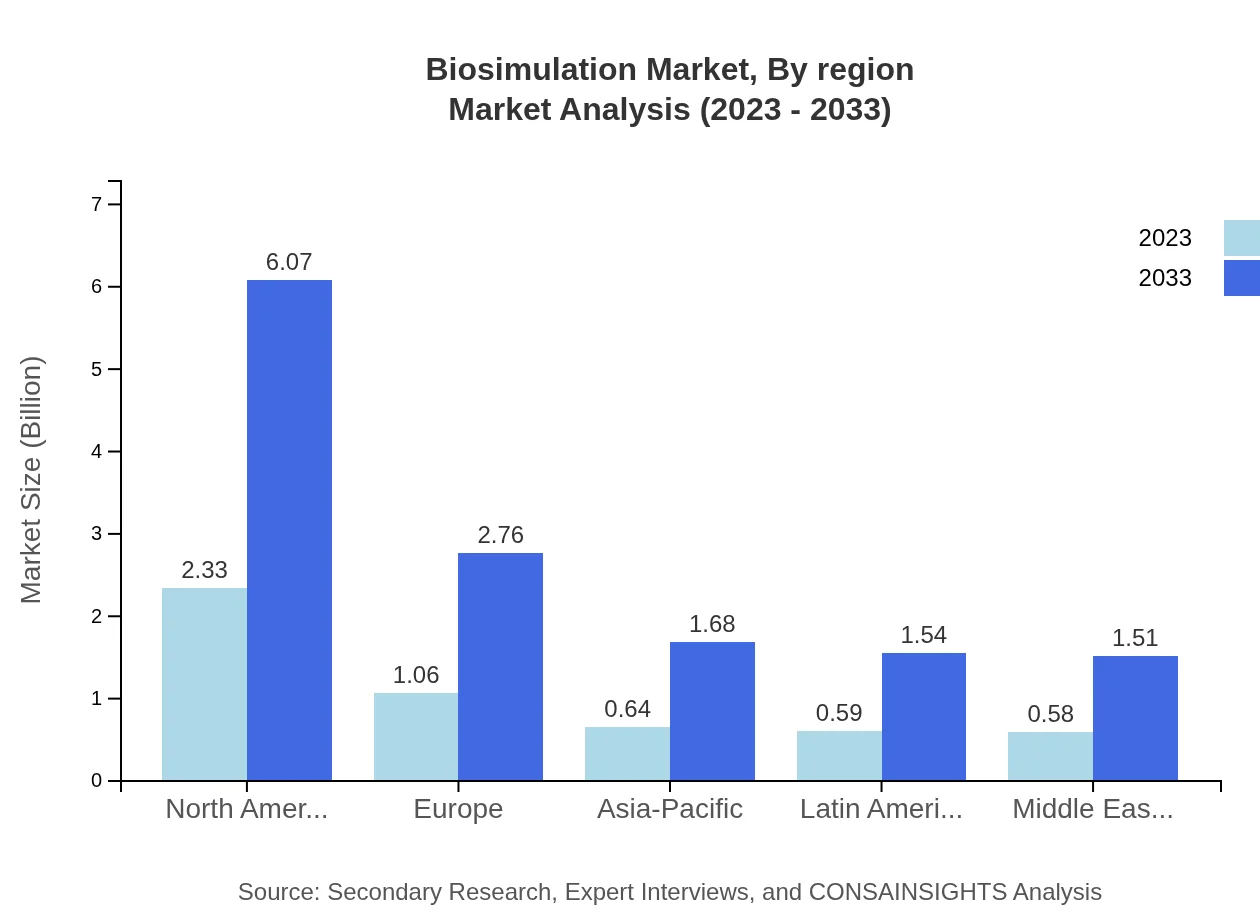
Biosimulation Market Analysis Report by Region
Europe Biosimulation Market Report:
Europe's market is projected to increase from USD 1.61 billion in 2023 to USD 4.19 billion by 2033. The region benefits from stringent regulatory frameworks backing the use of biosimulation in drug development, coupled with a strong focus on personalized medicine and patient-centric approaches to healthcare.Asia Pacific Biosimulation Market Report:
The Asia Pacific biosimulation market is projected to grow from USD 0.93 billion in 2023 to USD 2.44 billion by 2033. This growth is driven by rising investments in biotechnology and pharmaceutical research, increasing need for predictive modeling in drug development, and expanding healthcare infrastructure. Countries like China and India are becoming hubs for biosimulation research.North America Biosimulation Market Report:
The North American biosimulation market is leading globally, anticipated to expand from USD 1.96 billion in 2023 to USD 5.10 billion by 2033. High levels of investment in R&D by major pharmaceutical firms, robust healthcare regulations facilitating the adoption of innovative technologies, and a well-established biotechnology sector underlie this growth.South America Biosimulation Market Report:
In South America, the market is expected to transition from USD 0.30 billion in 2023 to USD 0.78 billion in 2033. The growth is facilitated by increasing collaborations between local biopharma companies and universities, as well as government initiatives aimed at fostering research in drug development.Middle East & Africa Biosimulation Market Report:
In the Middle East and Africa, the biosimulation market is poised to grow from USD 0.41 billion in 2023 to USD 1.06 billion by 2033, driven by the increasing adoption of advanced technologies in healthcare sectors and efforts to establish R&D centers focused on medication and treatment innovations.Tell us your focus area and get a customized research report.
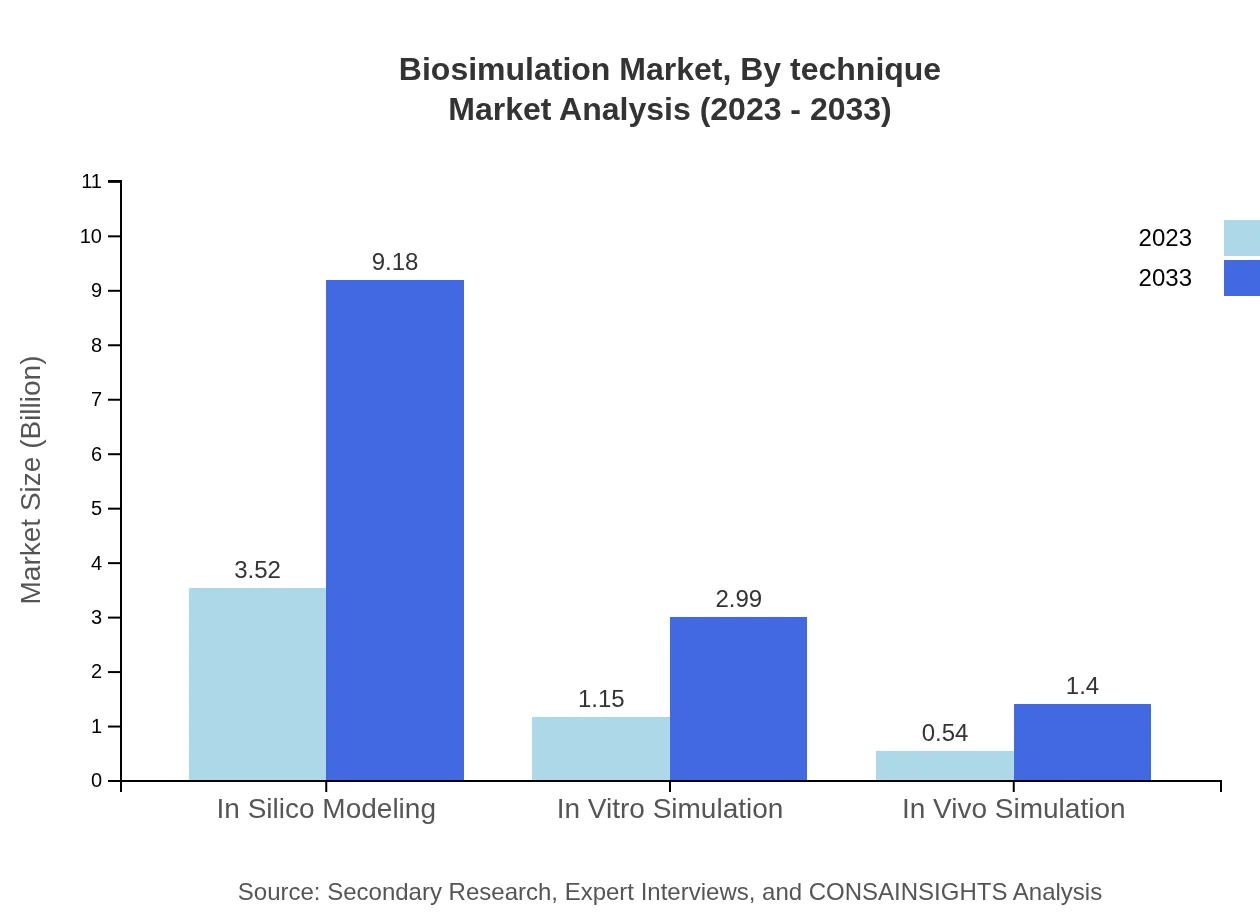
Biosimulation Market Analysis By Technique
The technique segment encompasses in silico modeling, in vitro simulation, and in vivo simulation approaches. In silico modeling dominates the market, representing 67.66% share due to its applications in drug discovery processes. In vitro and in vivo simulations, while smaller at 22.05% and 10.29% respectively, are vital for testing biological impact and toxicity of compounds.
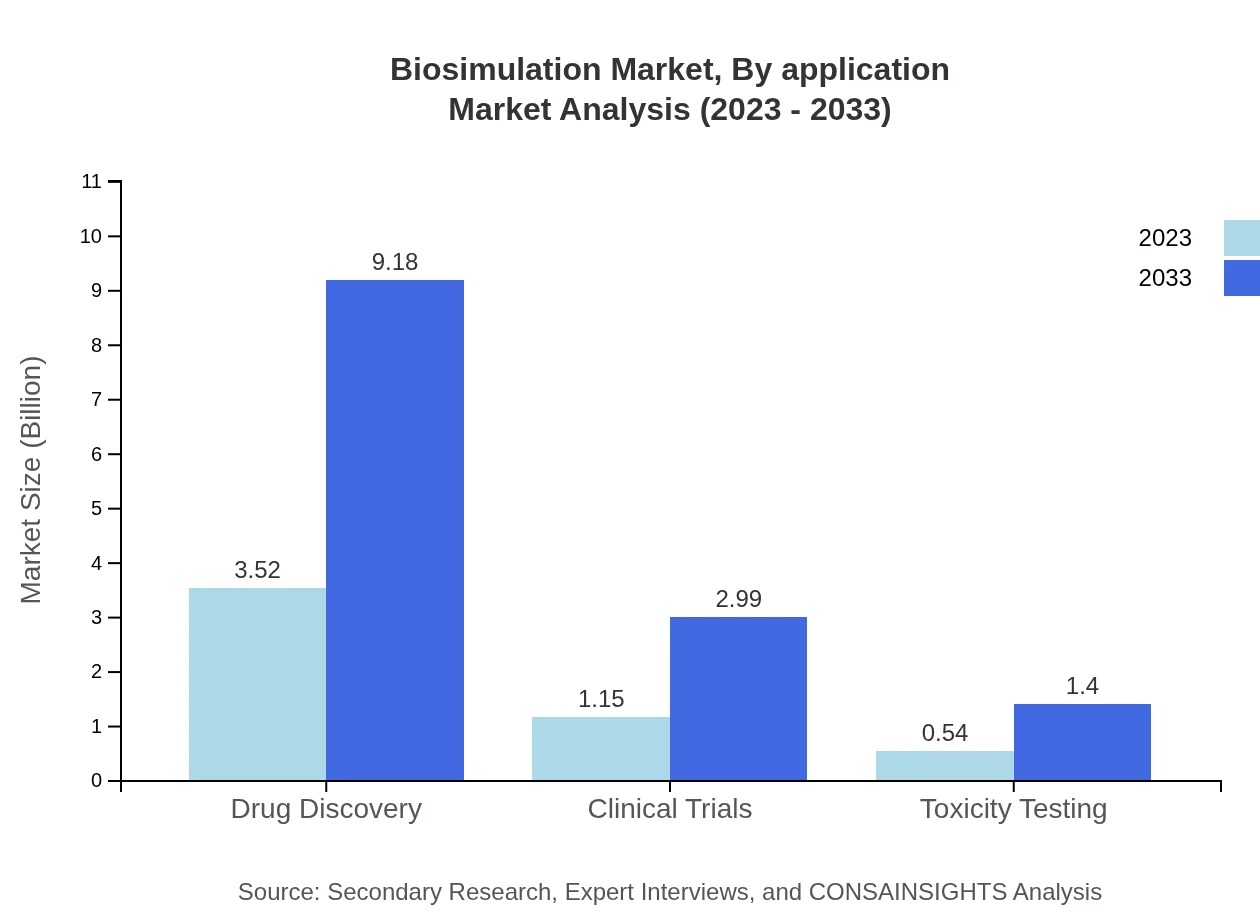
Biosimulation Market Analysis By Application
Drug discovery leads this segment, holding a share of 67.66% due to its integration in initial drug development phases. Clinical trials and toxicity testing contribute with shares of 22.05% and 10.29%, respectively, reflecting their crucial roles in validating drug efficacy and safety.
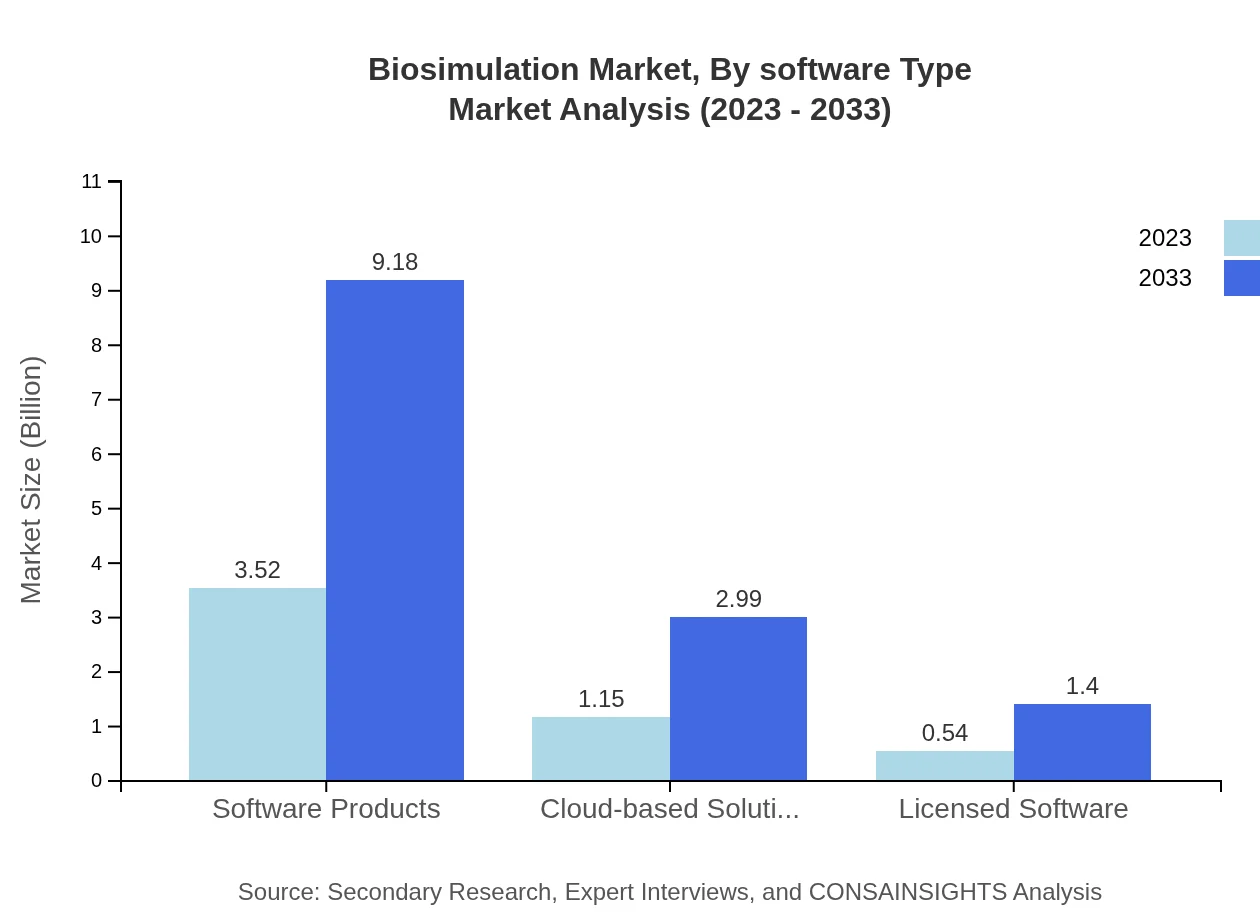
Biosimulation Market Analysis By Software Type
Software products represent the bulk of the market at 67.66%, focusing on in silico modeling applications. Cloud-based solutions are gaining traction, accounting for 22.05% of the share as they offer flexible, scalable options for biotech companies, while licensed software comprises 10.29%.
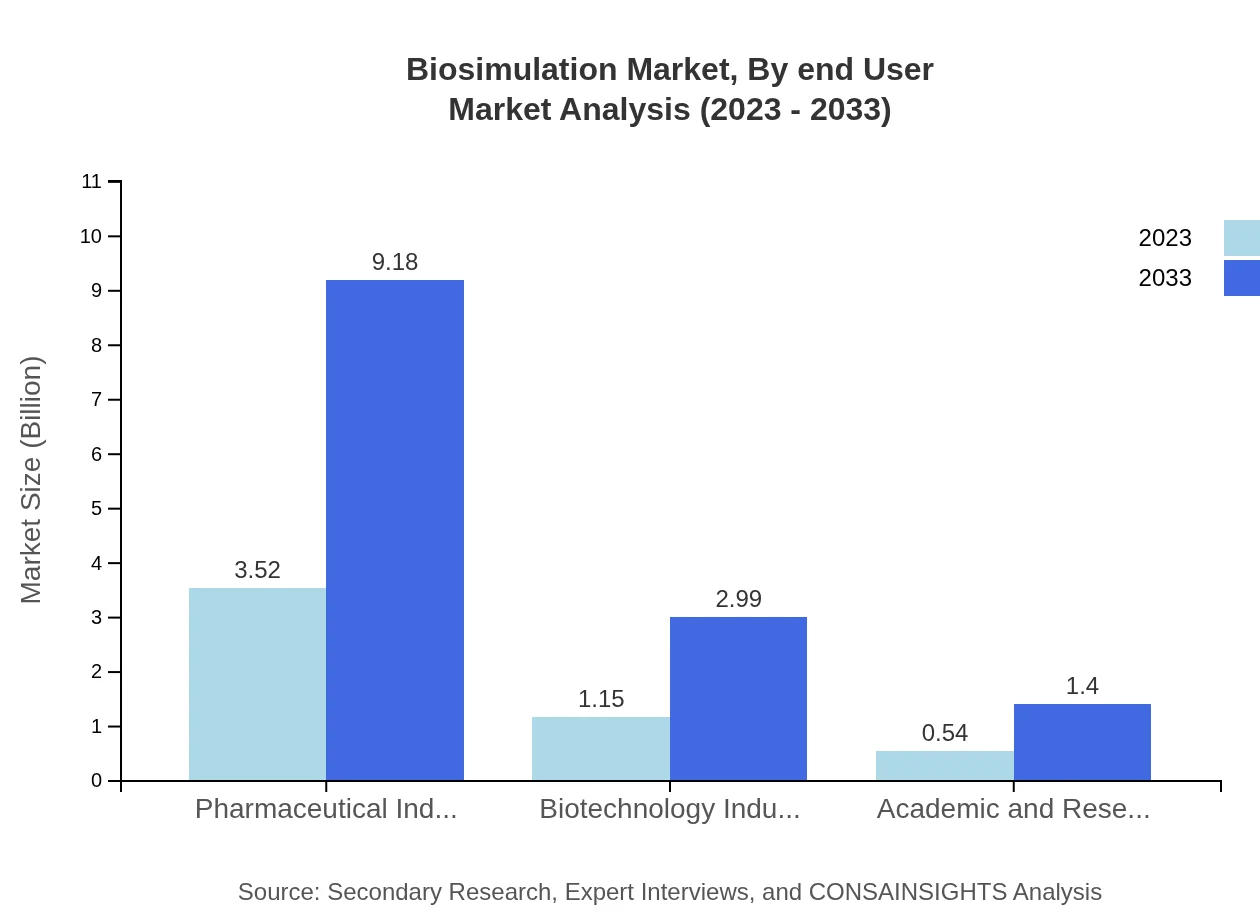
Biosimulation Market Analysis By End User
The pharmaceutical industry is the largest end-user, owning 67.66% of the market share as companies leverage biosimulation for drug development. The biotechnology sector accounts for 22.05%, while academic and research institutes hold 10.29% of the market, allowing for educational and foundational research applications.
Biosimulation Market Analysis By Region
Regional analysis shows North America as the market leader at 44.73% share, driven by innovation and R&D investments. Europe follows with 20.37% share, emphasizing regulatory acceptance. Asia Pacific and Latin America contribute with 12.38% and 11.36% shares respectively as emerging markets ramp up investment in health technologies.
Biosimulation Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Biosimulation Industry
Certara:
Certara is a leading biosimulation company providing cutting-edge modeling and simulation software, enhancing drug development across FDA-regulated industries.Simulations Plus:
Simulations Plus specializes in software for drug discovery and development, leveraging advanced modeling technologies to predict pharmacokinetics and pharmacodynamics.Rhenovia Pharma:
Rhenovia Pharma focuses on creating computational modeling tools for drug discovery and development, contributing significantly to the biosimulation landscape.Insilico Medicine:
Insilico Medicine utilizes AI and machine learning in biosimulation, providing insights for drug development and personalized therapies.We're grateful to work with incredible clients.









FAQs
What is the market size of biosimulation?
As of 2023, the biosimulation market is valued at approximately $5.2 billion and is projected to grow at a compound annual growth rate (CAGR) of 9.7% over the coming years, suggesting robust expansion opportunities.
What are the key market players or companies in the biosimulation industry?
Key players in the biosimulation industry include major pharmaceutical and biotechnology companies such as Certara, Simulations Plus, and BIOVIA, among others. These organizations lead through innovation in computational modeling and simulation technologies.
What are the primary factors driving the growth in the biosimulation industry?
The growth of the biosimulation industry is driven by factors such as increased demand for drug discovery and development efficiency, rising R&D expenditures in the pharmaceutical sector, and regulatory requirements for predictive modeling that support the development of safe drugs.
Which region is the fastest Growing in the biosimulation market?
Among the various regions, North America is the fastest-growing in the biosimulation market, with an anticipated increase from $1.96 billion in 2023 to $5.10 billion by 2033, reflecting strong investment in innovative healthcare solutions.
Does ConsaInsights provide customized market report data for the biosimulation industry?
Yes, ConsaInsights specializes in providing customized market report data tailored to meet the specific needs of clients in the biosimulation industry, ensuring insights are relevant and actionable for strategic decision-making.
What deliverables can I expect from this biosimulation market research project?
From the biosimulation market research project, expect comprehensive reports detailing market size, growth trends, competitive analysis, segment performance, regional insights, and forecasts that will help in strategic planning and investment.
What are the market trends of biosimulation?
Current trends in the biosimulation market include the increasing use of in silico models for drug discovery, growth in personalized medicine, and developments in regulatory frameworks that encourage the adoption of advanced simulation technologies.