Blood Brain Barrier Technologies Market Report
Published Date: 31 January 2026 | Report Code: blood-brain-barrier-technologies
Blood Brain Barrier Technologies Market Size, Share, Industry Trends and Forecast to 2033
This report offers a comprehensive analysis of the Blood Brain Barrier Technologies market, detailing market size, growth forecasts, regional insights, and segment performance from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
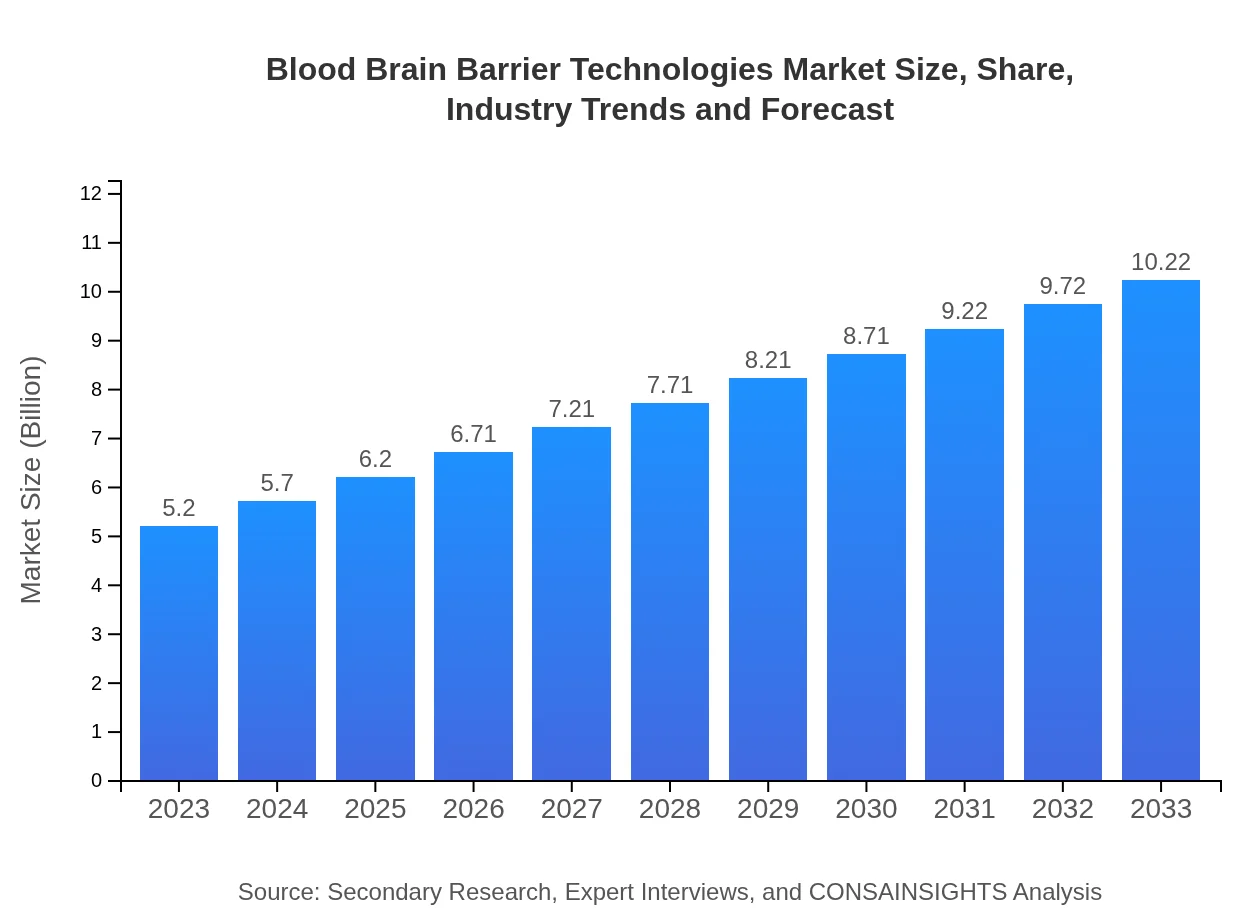
| 2023 Market Size | $5.20 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $10.22 Billion |
| Top Companies | Alchemia Limited, AbbVie Inc. |
| Last Modified Date | 31 January 2026 |
Blood Brain Barrier Technologies Market Overview
Customize Blood Brain Barrier Technologies Market Report market research report
- ✔ Get in-depth analysis of Blood Brain Barrier Technologies market size, growth, and forecasts.
- ✔ Understand Blood Brain Barrier Technologies's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Blood Brain Barrier Technologies
What is the Market Size & CAGR of Blood Brain Barrier Technologies market in 2033?
Blood Brain Barrier Technologies Industry Analysis
Blood Brain Barrier Technologies Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Blood Brain Barrier Technologies Market Analysis Report by Region
Europe Blood Brain Barrier Technologies Market Report:
The European market for Blood Brain Barrier Technologies is anticipated to grow from $1.66 billion in 2023 to $3.26 billion in 2033. The region benefits from advanced healthcare systems and robust pharmaceutical industries, particularly for CNS disorders.Asia Pacific Blood Brain Barrier Technologies Market Report:
The Asia-Pacific region is projected to exhibit the highest growth in the Blood Brain Barrier Technologies market, expanding from $1.02 billion in 2023 to $2.01 billion by 2033. This growth is attributed to a rise in healthcare spending, coupled with an increase in neurological disorders, which fuels demand for innovative treatment solutions.North America Blood Brain Barrier Technologies Market Report:
North America remains a significant market, with projections indicating growth from $1.73 billion in 2023 to $3.41 billion in 2033. The presence of major pharmaceutical firms and extensive R&D activities, supported by favorable reimbursement policies, drive this market.South America Blood Brain Barrier Technologies Market Report:
In South America, the market is expected to grow modestly from $0.13 billion in 2023 to $0.26 billion in 2033. Enhanced awareness regarding neurological diseases and increasing investments in healthcare infrastructure are key growth drivers in this region.Middle East & Africa Blood Brain Barrier Technologies Market Report:
In the Middle East and Africa, the market is projected to increase from $0.65 billion in 2023 to $1.28 billion in 2033. Growing awareness and an increasing focus on healthcare delivery innovations are driving growth in this region.Tell us your focus area and get a customized research report.
Blood Brain Barrier Technologies Market Analysis By Technology
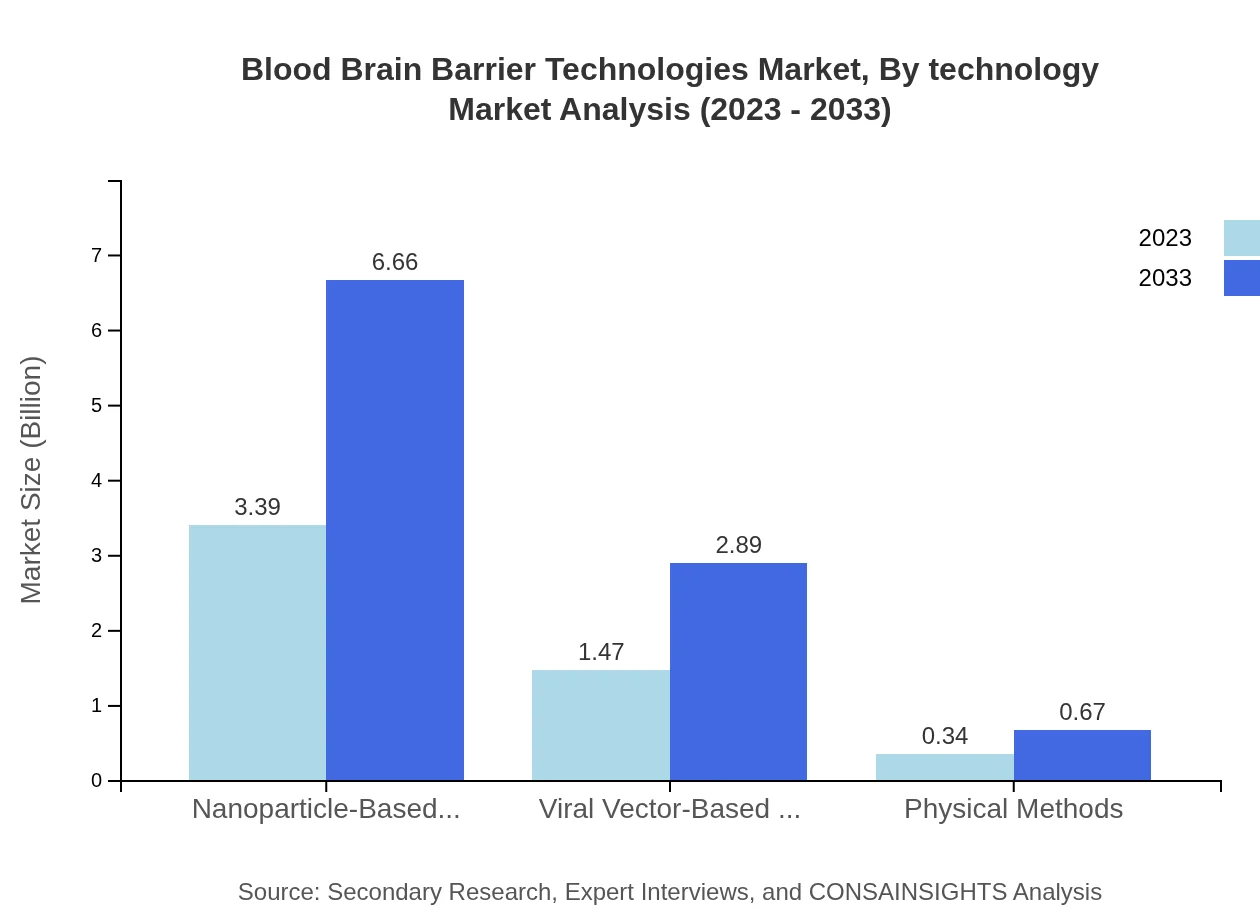
The Blood Brain Barrier Technologies market can be categorized by technology into nanoparticle-based delivery, viral vector-based delivery, and physical methods. Nanoparticle-based delivery dominates the market, reflecting a share of 65.19% in 2023, projected to maintain its leadership in 2033. Viral vector-based delivery also shows substantial growth, rising from $1.47 billion in 2023 to $2.89 billion by 2033, with an increasing share of 28.28%.
Blood Brain Barrier Technologies Market Analysis By Application
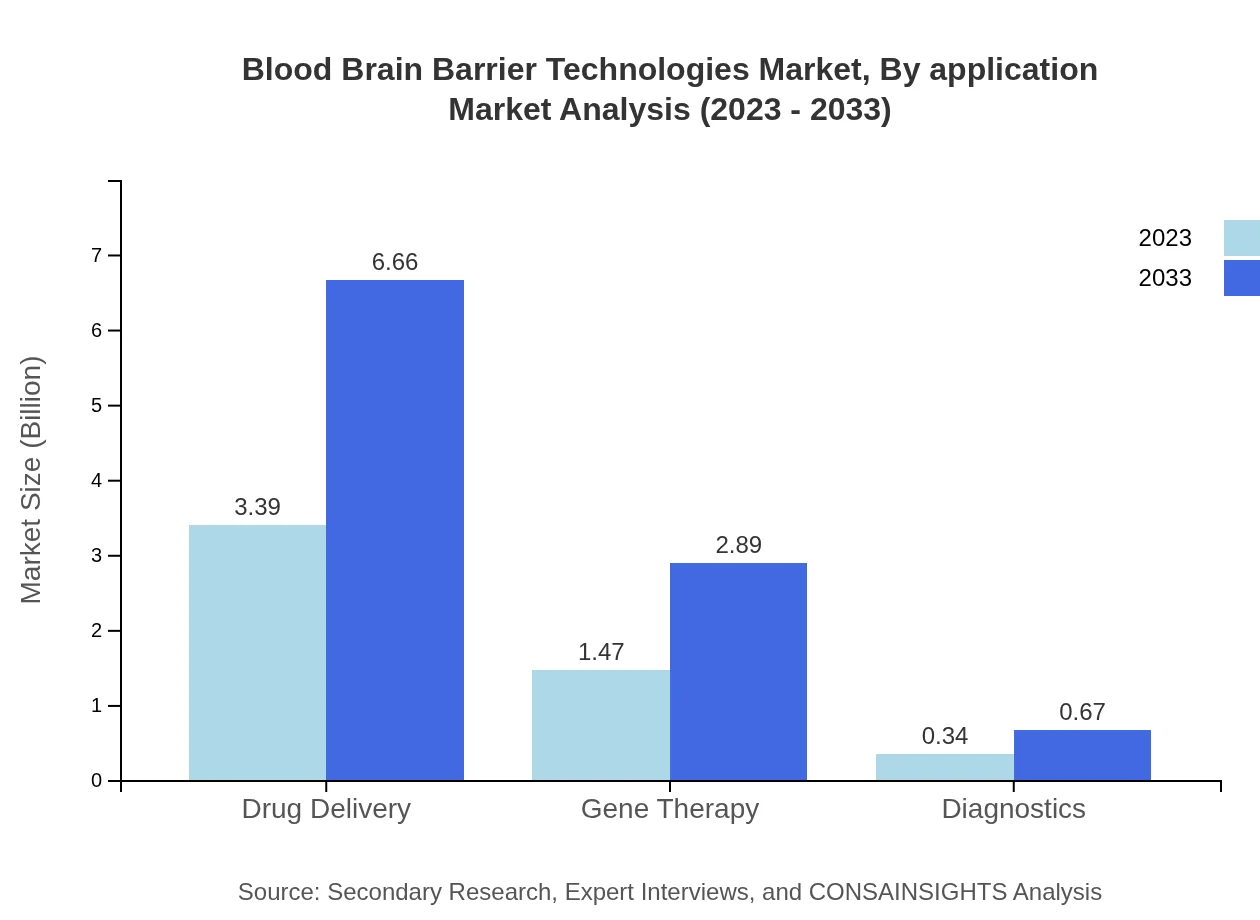
Different applications such as drug delivery, gene therapy, and diagnostics play a significant role in the market. Drug delivery holds the majority share, forecasted to remain at 65.19% throughout the next decade. Gene therapy and diagnostic applications are also expanding, with forecasts showing significant increases by 2033.
Blood Brain Barrier Technologies Market Analysis By End User
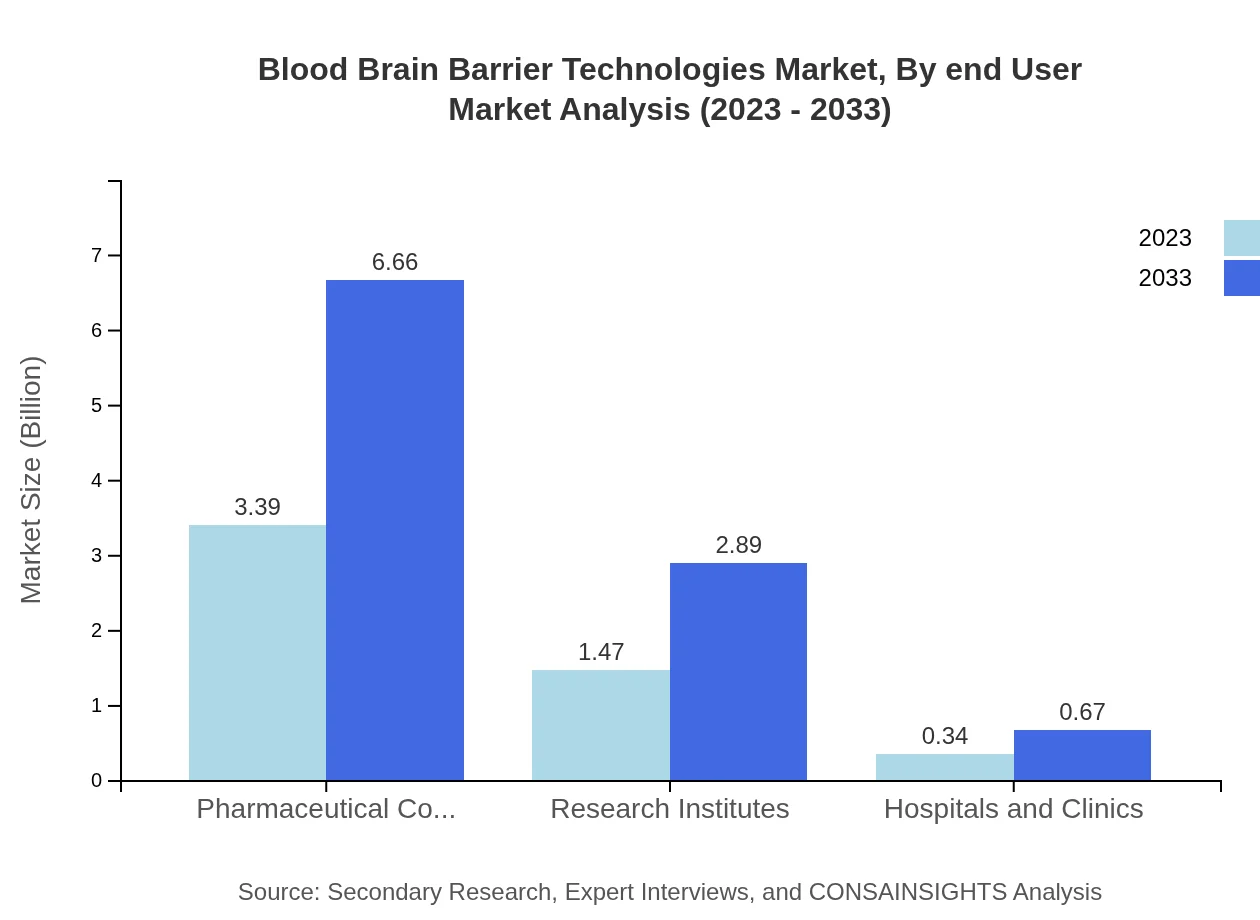
The key end-users in the BBB technologies market include pharmaceutical companies, research institutes, and hospitals/clinics. Pharmaceutical companies represent the largest segment with a market share of 65.19% in 2023 and expected growth, while research institutes and hospitals/clinics will perform steadily, contributing to the expanding demand for BBB Technologies.
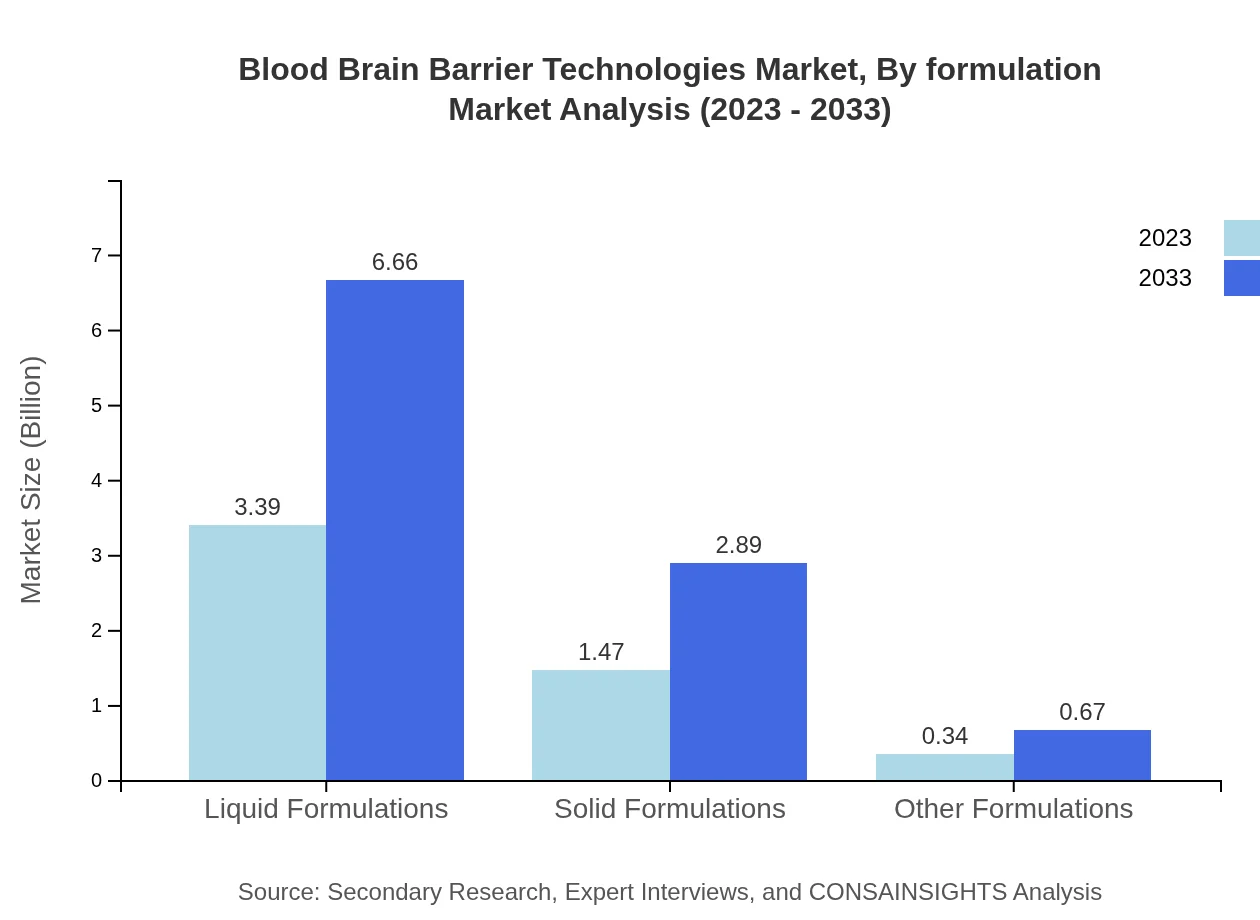
Blood Brain Barrier Technologies Market Analysis By Formulation
Formulations used include liquid, solid, and other formulations. Liquid formulations dominate the market, holding a share of 65.19% in 2023 while showing promises for future applications. Solid formulations are also significant with notable growth projected.
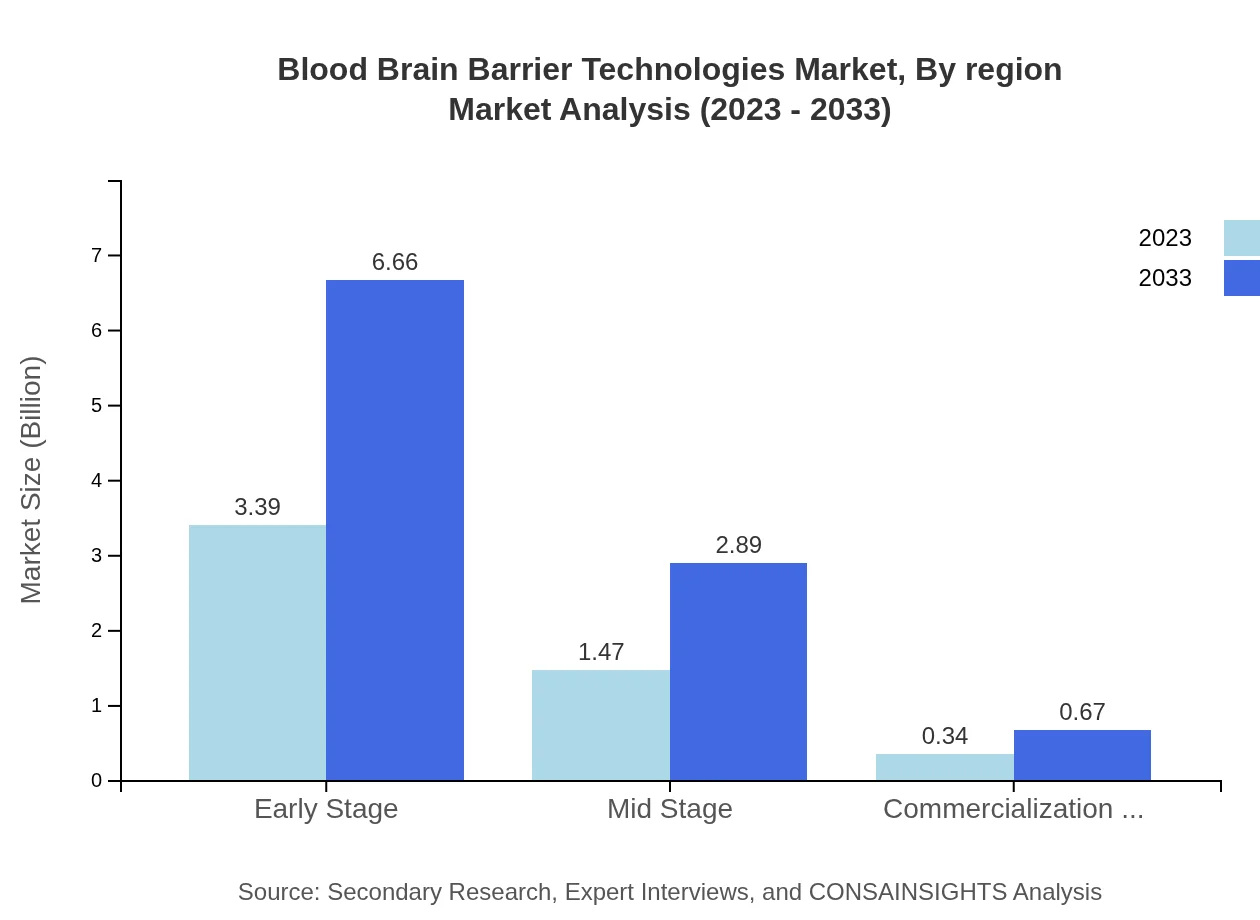
Blood Brain Barrier Technologies Market Analysis By Region
Significant interest is noted in early-stage and mid-stage technologies. Early-stage products command a size of $3.39 billion in 2023 and are projected to nearly double by 2033. Mid-stage technologies also show notable growth with varied applications and promising outcomes for commercial viability.
Blood Brain Barrier Technologies Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Blood Brain Barrier Technologies Industry
Alchemia Limited:
Focused on developing drug delivery systems specifically designed to cross the BBB, using proprietary technologies to enable more effective treatment options.AbbVie Inc.:
A leading player in the pharmaceutical industry, AbbVie is investing in advanced delivery technologies to enhance CNS therapy efficacy.We're grateful to work with incredible clients.









FAQs
What is the market size of blood Brain Barrier Technologies?
The blood-brain barrier technologies market is valued at approximately $5.2 billion in 2023, with a projected CAGR of 6.8%. By 2033, the market is expected to expand significantly, reflecting advancements in drug delivery methods and therapeutic solutions.
What are the key market players or companies in the blood Brain Barrier Technologies industry?
Key players in the blood-brain barrier technologies market include prominent pharmaceutical companies and research institutes focusing on innovative drug delivery systems. Their collaborations drive research and commercialization efforts, enhancing treatment options for neurological disorders.
What are the primary factors driving the growth in the blood Brain Barrier Technologies industry?
Growth drivers for the blood-brain barrier technologies market include increasing demand for effective drug delivery systems to treat CNS disorders, rising R&D investments in neurological therapies, and technological innovations in drug formulation and delivery methods.
Which region is the fastest Growing in the blood Brain Barrier Technologies?
The Asia Pacific region is poised as the fastest-growing area in the blood-brain barrier technologies market. With a projected increase from $1.02 billion in 2023 to $2.01 billion by 2033, it reflects a robust investment in healthcare and biotechnology facilities.
Does ConsaInsights provide customized market report data for the blood Brain Barrier Technologies industry?
Yes, ConsaInsights offers customized market report data tailored to client needs in the blood-brain barrier technologies industry. Clients can request specific insights, trends, and forecasts that align with their strategic objectives.
What deliverables can I expect from this blood Brain Barrier Technologies market research project?
Deliverables from the blood-brain barrier technologies market research project typically include comprehensive market analysis reports, detailed segmentation data, competitor analysis, growth forecasts, and strategic recommendations across various applications and regions.
What are the market trends of blood Brain Barrier Technologies?
Current trends in the blood-brain barrier technologies market include increasing use of nanoparticle-based delivery systems, innovative approaches to gene therapy, advancements in diagnostics, and a growing focus on personalized medicine to enhance treatment efficacy.