Blood Disorder Therapeutics Market Report
Published Date: 31 January 2026 | Report Code: blood-disorder-therapeutics
Blood Disorder Therapeutics Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Blood Disorder Therapeutics market from 2023 to 2033, covering market size, trends, and forecasts. It aims to offer insights into the competitive landscape, technological advancements, and segmentation within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
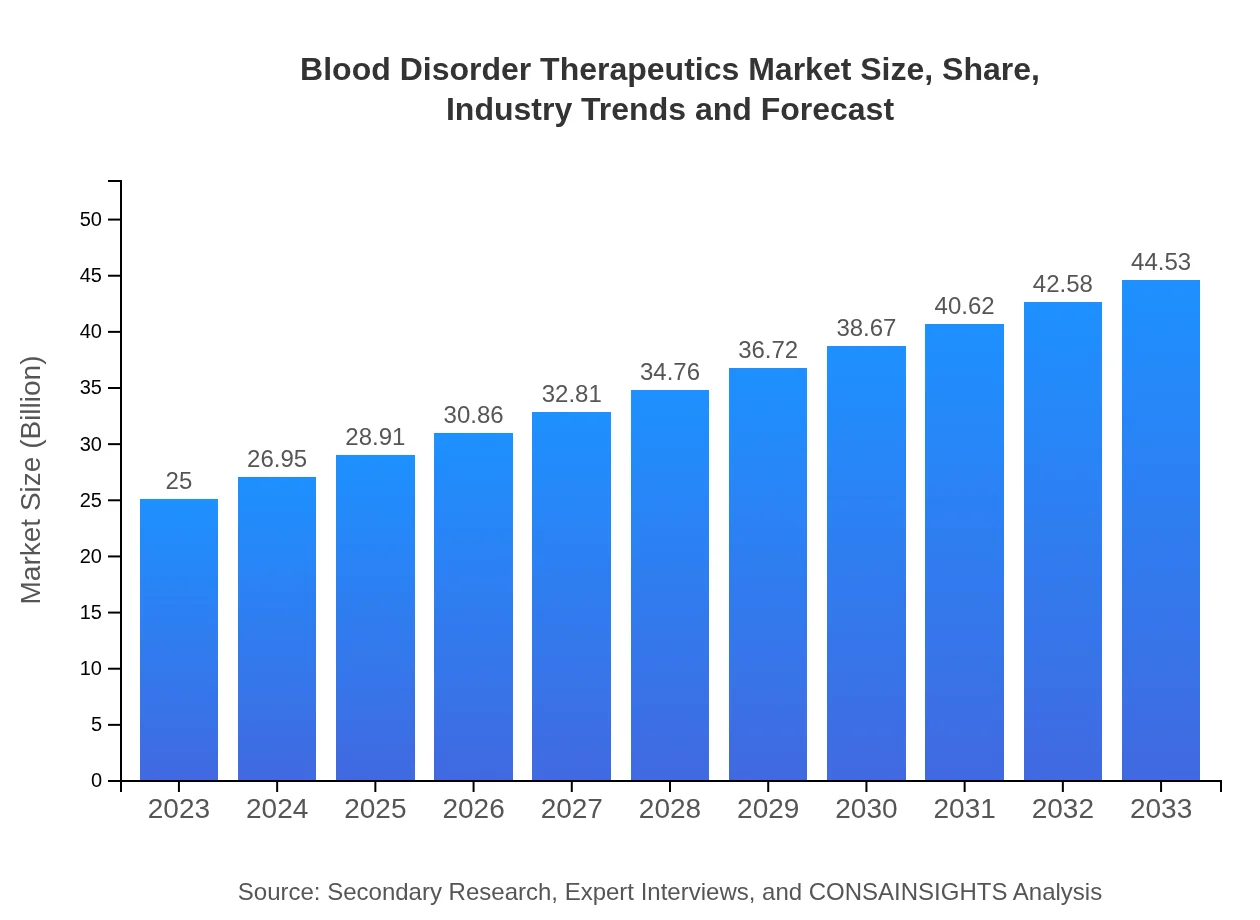
| 2023 Market Size | $25.00 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $44.53 Billion |
| Top Companies | Roche, Bayer AG, Novo Nordisk, CSL Behring |
| Last Modified Date | 31 January 2026 |
Blood Disorder Therapeutics Market Overview
Customize Blood Disorder Therapeutics Market Report market research report
- ✔ Get in-depth analysis of Blood Disorder Therapeutics market size, growth, and forecasts.
- ✔ Understand Blood Disorder Therapeutics's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Blood Disorder Therapeutics
What is the Market Size & CAGR of Blood Disorder Therapeutics market in 2033?
Blood Disorder Therapeutics Industry Analysis
Blood Disorder Therapeutics Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Blood Disorder Therapeutics Market Analysis Report by Region
Europe Blood Disorder Therapeutics Market Report:
In Europe, the Blood Disorder Therapeutics market size is expected to rise from $6.62 billion in 2023 to $11.79 billion by 2033, driven by well-established healthcare systems and increasing emphasis on innovative therapies.Asia Pacific Blood Disorder Therapeutics Market Report:
The Asia Pacific region is experiencing rapid growth in the Blood Disorder Therapeutics market, with a projected market size of $9.41 billion by 2033, up from $5.28 billion in 2023. Increasing healthcare expenditure and a rise in chronic diseases are propelling market growth in countries like China and India.North America Blood Disorder Therapeutics Market Report:
North America holds a significant market share, projected to grow from $9.31 billion in 2023 to $16.59 billion in 2033. The region benefits from advanced healthcare technology, substantial R&D investments, and a high prevalence of blood disorders.South America Blood Disorder Therapeutics Market Report:
South America is expected to show a moderate growth in the Blood Disorder Therapeutics market, reaching $1.82 billion by 2033 from $1.02 billion in 2023. Factors such as improving healthcare infrastructure and rising awareness about blood disorders drive this growth.Middle East & Africa Blood Disorder Therapeutics Market Report:
The Middle East and Africa are also predicted to see growth from $2.76 billion in 2023 to $4.92 billion by 2033. The market expansion is fueled by improving health services and enhanced access to therapies.Tell us your focus area and get a customized research report.
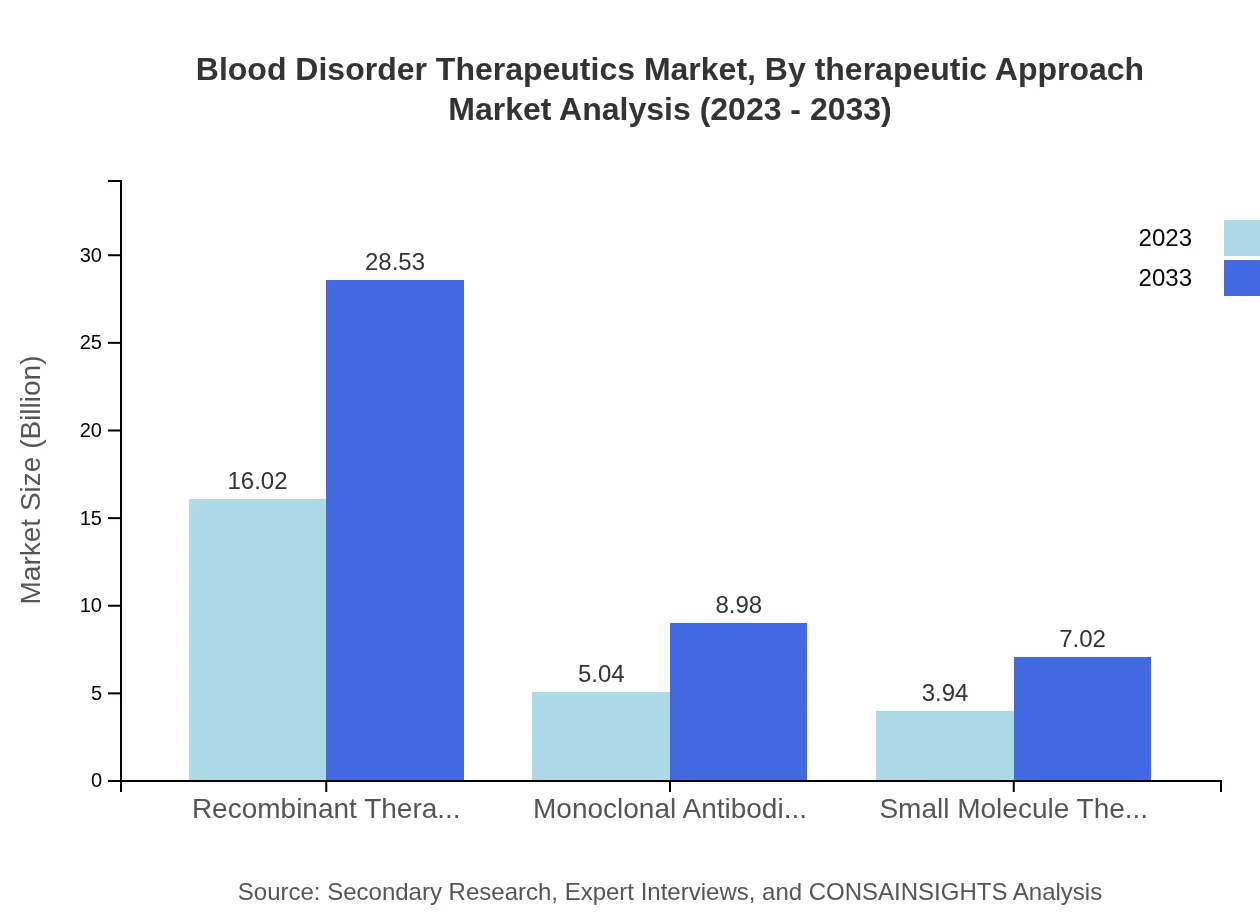
Blood Disorder Therapeutics Market Analysis By Therapeutic Approach
The therapeutic approach segment, which includes recombinant therapies, monoclonal antibodies, and small molecule therapies, plays a crucial role in treatment efficacy. Recombinant therapies dominate the market, accounting for a significant share due to their effectiveness in treating hemophilia, with a market size expected to grow from $16.02 billion in 2023 to $28.53 billion in 2033.
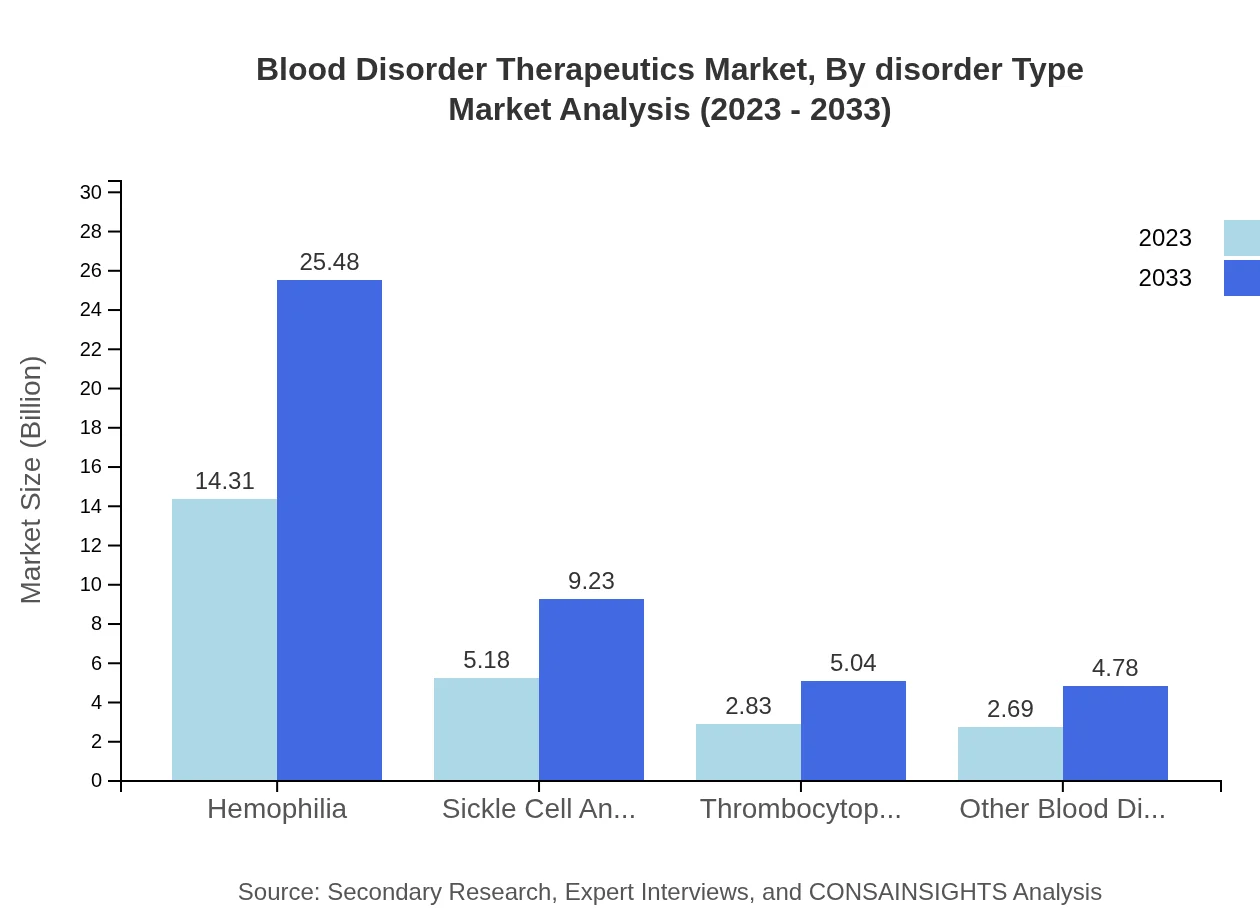
Blood Disorder Therapeutics Market Analysis By Disorder Type
The disorder type segment is crucial, with hemophilia leading the market, showcasing growth from $14.31 billion in 2023 to $25.48 billion in 2033. Sickle cell anemia and thrombocytopenia also represent important sub-segments, expected to maintain strong growth due to increasing incidences and improved treatment options.
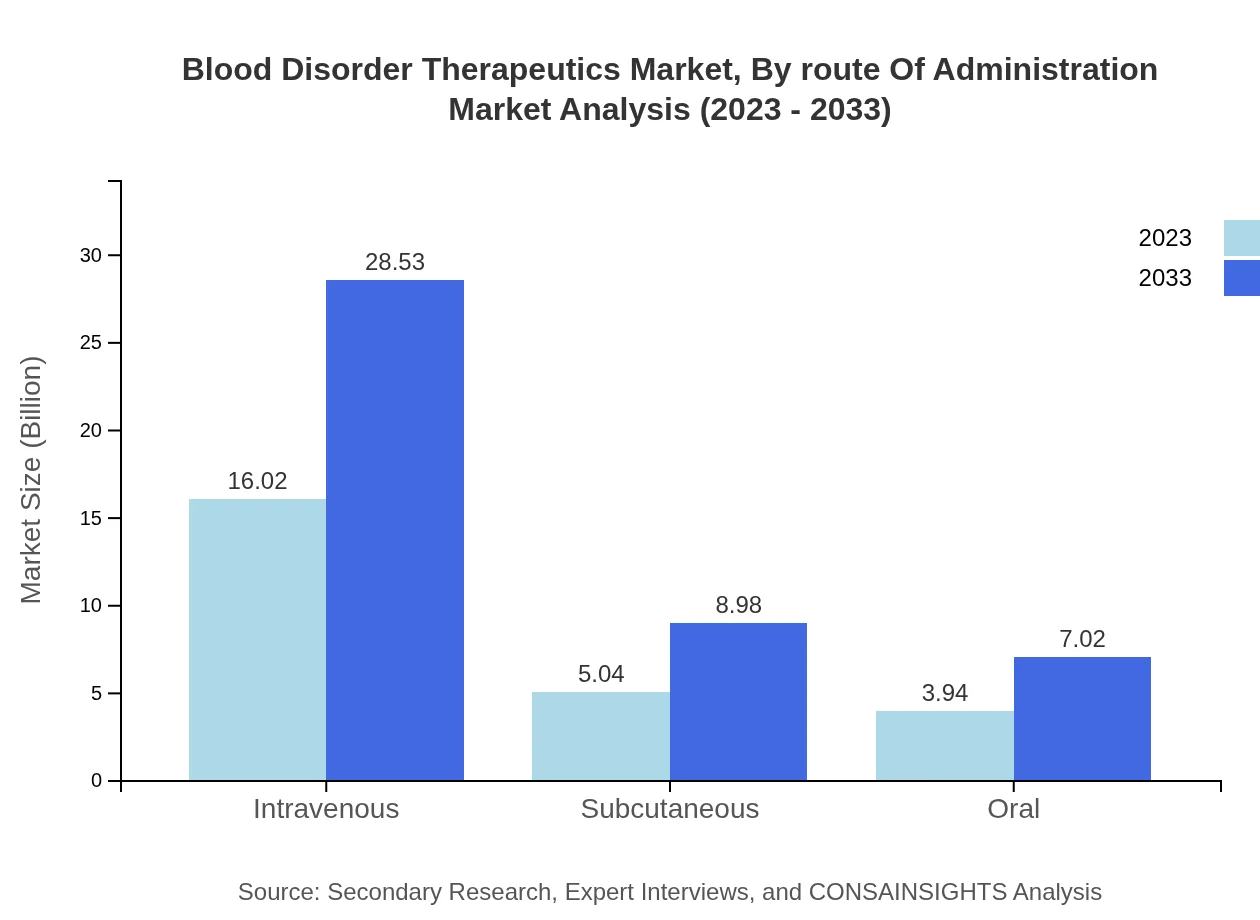
Blood Disorder Therapeutics Market Analysis By Route Of Administration
The market is segmented by route of administration, with intravenous therapies leading, projected to grow from $16.02 billion in 2023 to $28.53 billion in 2033. Subcutaneous and oral routes are gaining traction due to patient convenience and are crucial for future market innovation.
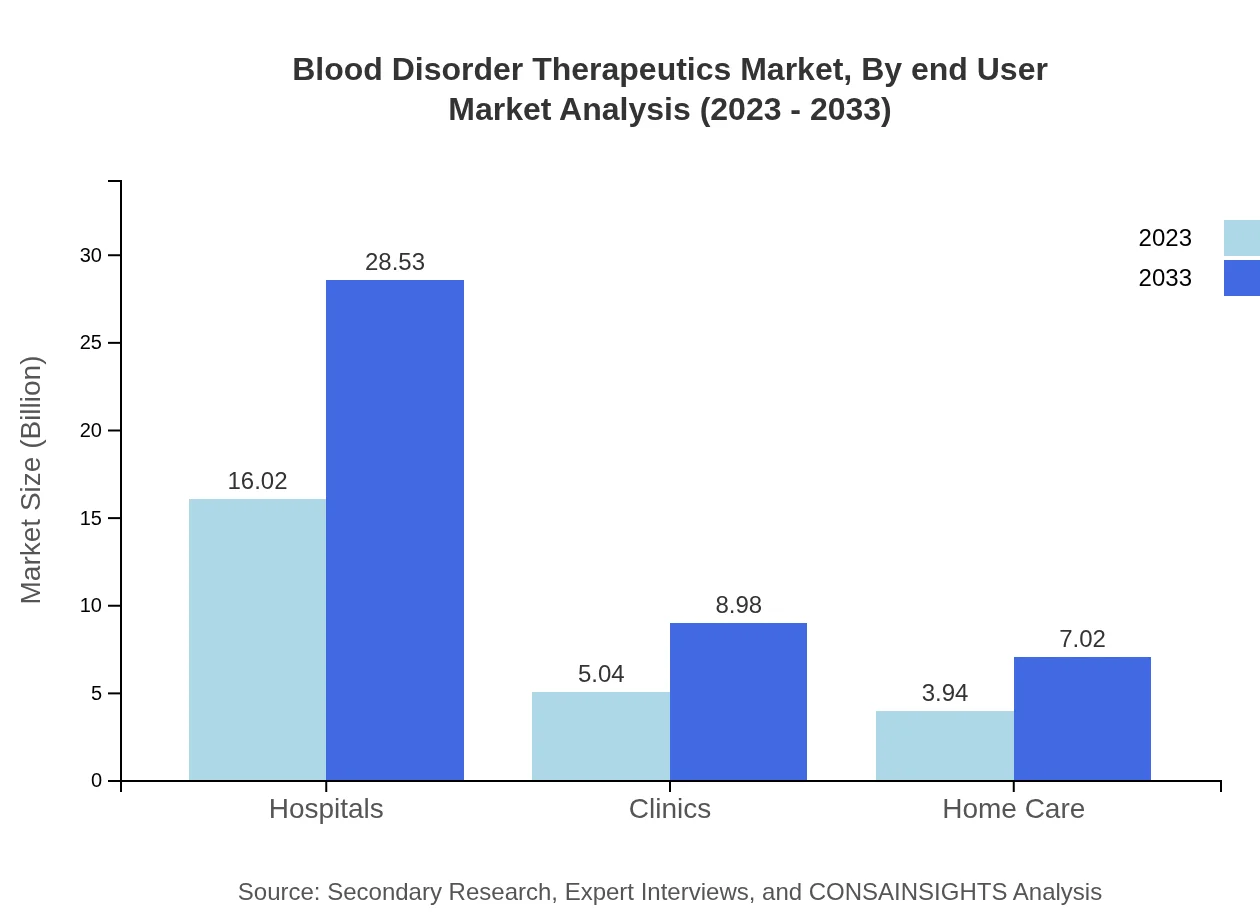
Blood Disorder Therapeutics Market Analysis By End User
Hospitals dominate the end-user segment, with a market share expected to remain steady at 64.06%. The growth of clinics and home care treatments, though smaller at 20.17% and 15.77% respectively, is significant as care models evolve toward outpatient and at-home therapies.
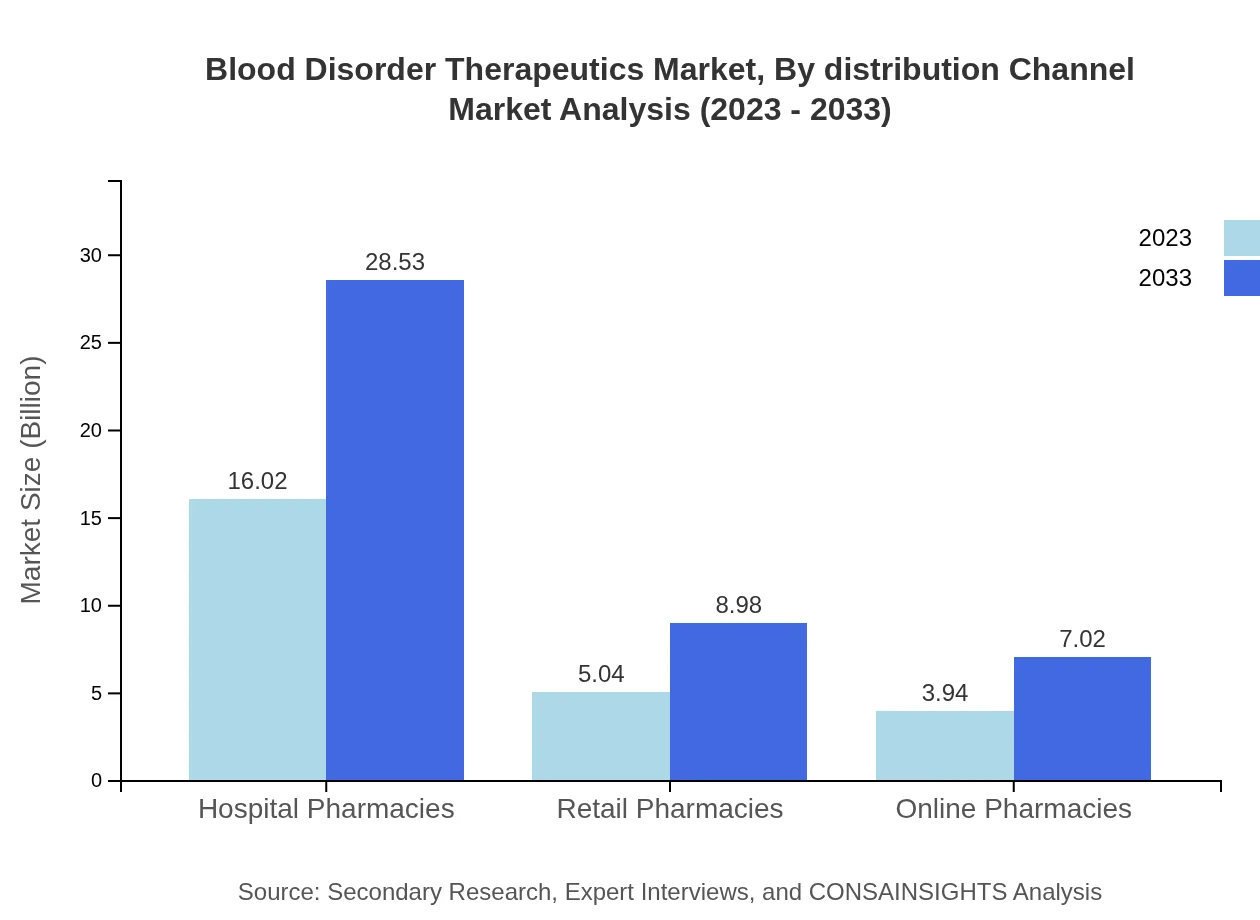
Blood Disorder Therapeutics Market Analysis By Distribution Channel
Distribution channels for the market include hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies hold the largest share (64.06%), while online pharmacies are becoming increasingly popular, growing from $3.94 billion in 2023 to $7.02 billion by 2033, driven by changes in consumer behavior.
Blood Disorder Therapeutics Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Blood Disorder Therapeutics Industry
Roche:
Roche is at the forefront of Blood Disorder Therapeutics, leading with innovative treatments for hemophilia and investing heavily in research for new therapies.Bayer AG:
Bayer AG focuses on developing comprehensive solutions for blood disorders, particularly in gene therapy and personalized medicine.Novo Nordisk:
Novo Nordisk offers a range of therapies focusing on hemophilia, with a commitment to patient education and healthcare improvements.CSL Behring:
CSL Behring is known for its specialized treatment options for bleeding disorders, including a wide array of immunoglobulin products.We're grateful to work with incredible clients.









FAQs
What is the market size of blood Disorder Therapeutics?
The global blood disorder therapeutics market was valued at approximately $25 billion in 2023 and is projected to grow at a CAGR of 5.8%, reaching near $38 billion by 2033.
What are the key market players or companies in the blood disorder therapeutics industry?
Key players in the blood disorder therapeutics market include major pharmaceutical companies such as Pfizer, Novartis, Roche, Teva Pharmaceutical Industries, and Bristol-Myers Squibb, which specialize in innovative therapies for blood disorders.
What are the primary factors driving the growth in the blood disorder therapeutics industry?
Factors driving growth include increased patient awareness, advancements in genetic therapies, rising prevalence of blood disorders like hemophilia and sickle cell anemia, and a surge in research funding dedicated to hematological treatments.
Which region is the fastest Growing in the blood disorder therapeutics?
North America is currently the fastest-growing region in the blood disorder therapeutics market, projected to expand from $9.31 billion in 2023 to $16.59 billion by 2033, driven by high healthcare expenditure and advanced treatment options.
Does Consainsights provide customized market report data for the blood disorder therapeutics industry?
Yes, Consainsights offers customized market report data tailored to the specific needs of clients in the blood disorder therapeutics industry, ensuring actionable insights based on unique market demands and trends.
What deliverables can I expect from this blood disorder therapeutics market research project?
Deliverables include comprehensive market analysis reports, growth forecasts, competitive landscape assessments, segmentation analysis, and strategic recommendations tailored to enhance decision-making in the blood disorder therapeutics sector.
What are the market trends of blood disorder therapeutics?
Market trends indicate a shift towards personalized medicine, increased adoption of home healthcare options, and a growing focus on gene therapies and biologics to treat various blood disorders effectively.