Breast Cancer Liquid Biopsy Market Report
Published Date: 31 January 2026 | Report Code: breast-cancer-liquid-biopsy
Breast Cancer Liquid Biopsy Market Size, Share, Industry Trends and Forecast to 2033
This report explores the Breast Cancer Liquid Biopsy market, providing insights on market dynamics, growth trends, and segmentation from 2023 to 2033, aimed at industry stakeholders and investors seeking to understand future opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
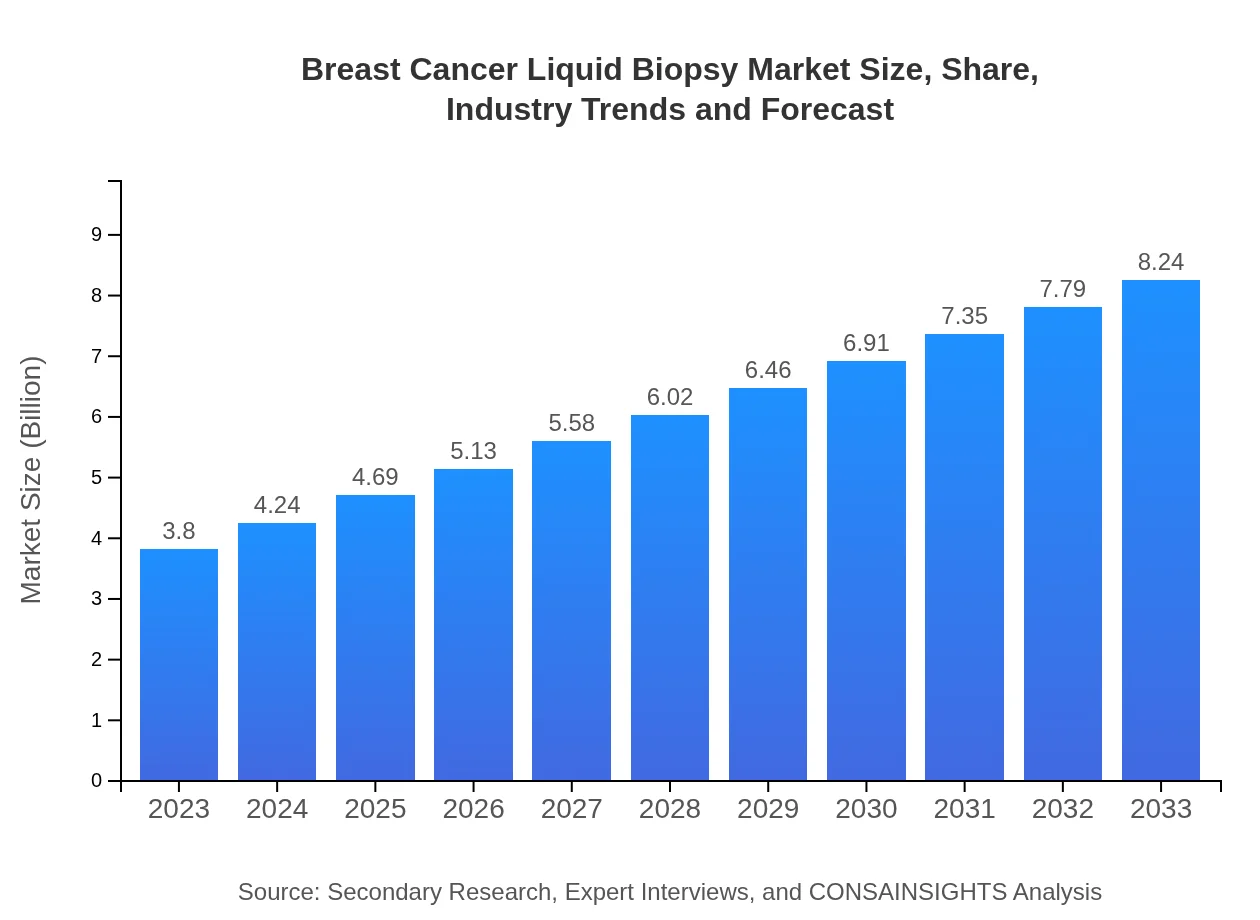
| 2023 Market Size | $3.80 Billion |
| CAGR (2023-2033) | 7.8% |
| 2033 Market Size | $8.24 Billion |
| Top Companies | Guardant Health, Sysmex Corporation, Roche |
| Last Modified Date | 31 January 2026 |
Breast Cancer Liquid Biopsy Market Overview
Customize Breast Cancer Liquid Biopsy Market Report market research report
- ✔ Get in-depth analysis of Breast Cancer Liquid Biopsy market size, growth, and forecasts.
- ✔ Understand Breast Cancer Liquid Biopsy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Breast Cancer Liquid Biopsy
What is the Market Size & CAGR of Breast Cancer Liquid Biopsy market in 2023?
Breast Cancer Liquid Biopsy Industry Analysis
Breast Cancer Liquid Biopsy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Breast Cancer Liquid Biopsy Market Analysis Report by Region
Europe Breast Cancer Liquid Biopsy Market Report:
European market size is expected to rise from USD 1.14 billion in 2023 to USD 2.47 billion in 2033. Rising investments in cancer research, along with supportive regulatory frameworks for liquid biopsy technologies, support market expansion in this region.Asia Pacific Breast Cancer Liquid Biopsy Market Report:
In Asia Pacific, the Breast Cancer Liquid Biopsy market is expected to grow from USD 0.83 billion in 2023 to USD 1.79 billion by 2033, driven by the rising prevalence of breast cancer and improvements in healthcare infrastructure. Increasing investment in biopharmaceutical research and growing awareness about early cancer detection also contribute to market growth in this region.North America Breast Cancer Liquid Biopsy Market Report:
North America holds the largest market share, projected to increase from USD 1.22 billion in 2023 to USD 2.64 billion by 2033. Strong reimbursement policies, a high prevalence of breast cancer, and the presence of key market players in the U.S. significantly bolster the market's growth.South America Breast Cancer Liquid Biopsy Market Report:
The South American market is projected to expand from USD 0.34 billion in 2023 to USD 0.75 billion by 2033. The growth is aided by an increase in breast cancer awareness and the gradual adoption of advanced diagnostic technologies, although market penetration remains lower than in developed regions.Middle East & Africa Breast Cancer Liquid Biopsy Market Report:
In the Middle East and Africa, the market is anticipated to grow from USD 0.27 billion in 2023 to USD 0.59 billion by 2033. Growth factors include an increasing focus on cancer diagnostics and the developing healthcare landscape, though challenges such as limited access to advanced diagnostic solutions persist.Tell us your focus area and get a customized research report.
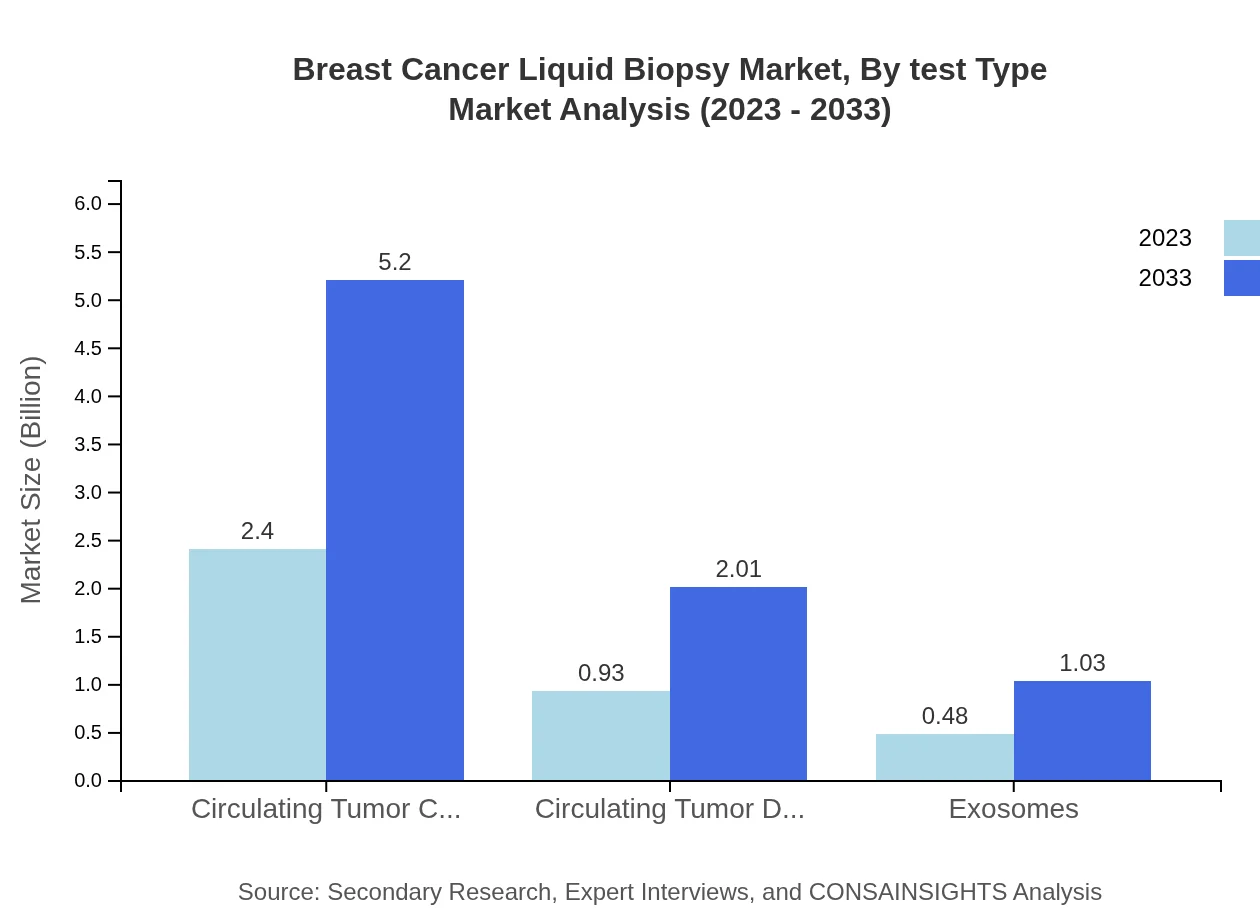
Breast Cancer Liquid Biopsy Market Analysis By Test Type
The Breast Cancer Liquid Biopsy market by test type is dominated by Circulating Tumor Cells (CTCs), contributing USD 2.40 billion in 2023 and expected to grow to USD 5.20 billion by 2033, holding a market share of 63.13%. Circulating Tumor DNA (ctDNA) follows closely, with a market size projected to expand from USD 0.93 billion to USD 2.01 billion, representing 24.35% of the market share by 2033.
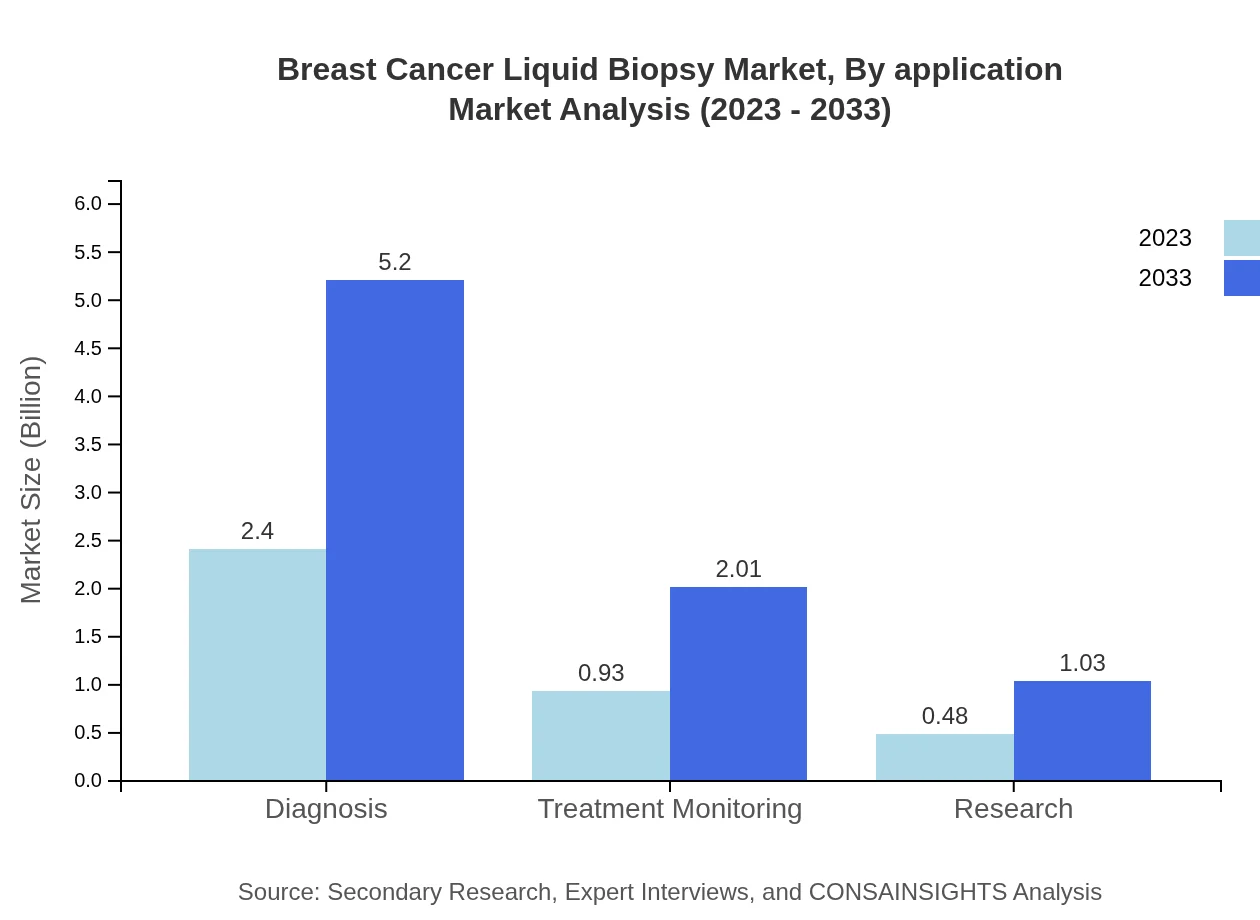
Breast Cancer Liquid Biopsy Market Analysis By Application
Applications in the Breast Cancer Liquid Biopsy market are mainly for diagnosis and treatment monitoring. The diagnosis segment is forecasted to grow from USD 2.40 billion in 2023 to USD 5.20 billion by 2033, maintaining 63.13% market share, while treatment monitoring is expected to increase from USD 0.93 billion to USD 2.01 billion, accounting for 24.35% share.
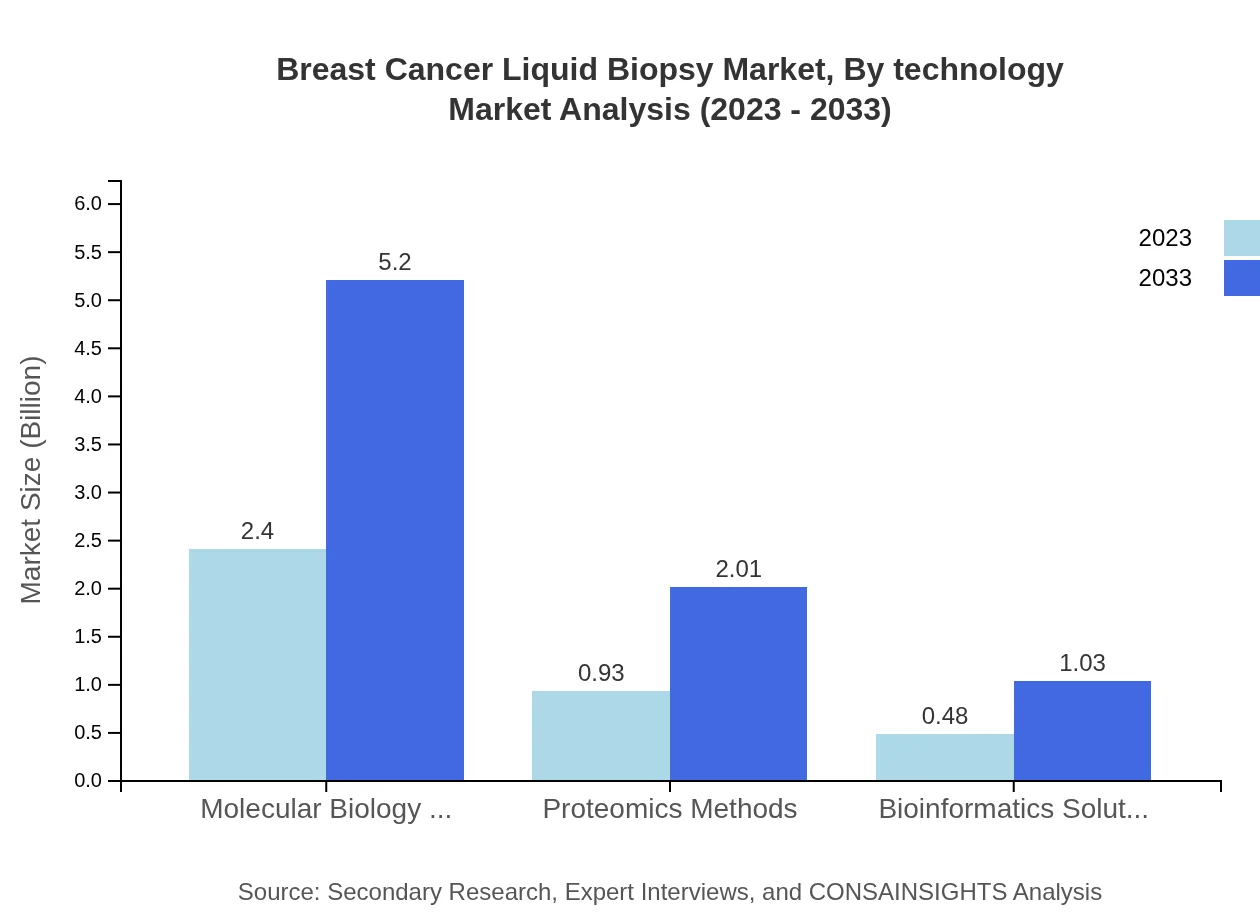
Breast Cancer Liquid Biopsy Market Analysis By Technology
Technological advancements play a critical role in the Breast Cancer Liquid Biopsy market. Molecular biology methods like PCR dominate with a market size of USD 2.40 billion in 2023, projected to increase to USD 5.20 billion by 2033. Next-Generation Sequencing (NGS) and bioinformatics solutions are gaining momentum, contributing to better accuracy in diagnostics.
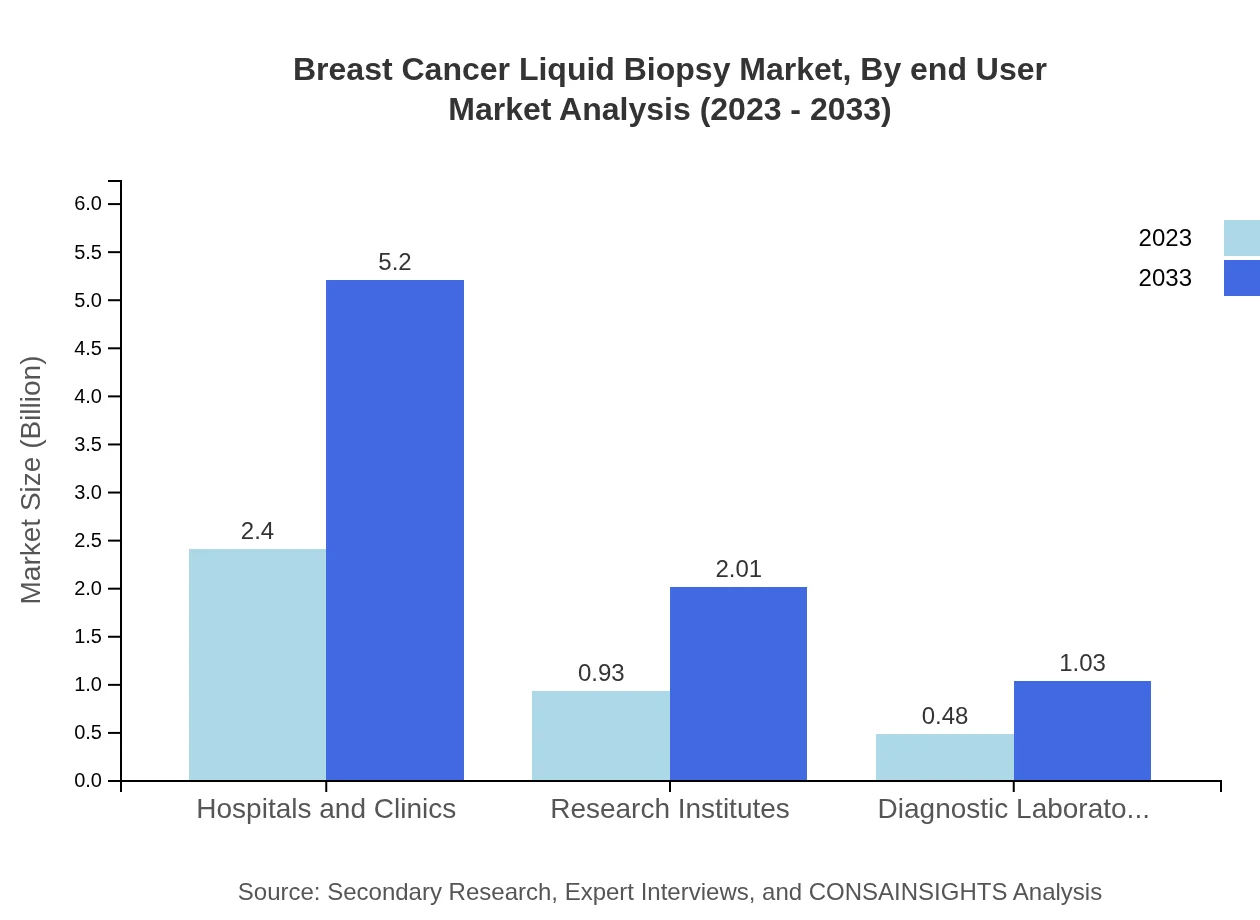
Breast Cancer Liquid Biopsy Market Analysis By End User
End-users of the Breast Cancer Liquid Biopsy market include hospitals and clinics, research institutes, and diagnostic laboratories. Hospitals hold the largest market share of 63.13%, valued at USD 2.40 billion in 2023 and expected to rise to USD 5.20 billion by 2033. Research institutes and diagnostic laboratories contribute significantly, making up 24.35% and 12.52% of the market share respectively.
Breast Cancer Liquid Biopsy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Breast Cancer Liquid Biopsy Industry
Guardant Health:
A leader in precision oncology, Guardant Health specializes in non-invasive genomic testing services that provide critical information for breast cancer treatment.Sysmex Corporation:
Sysmex develops innovative diagnostic solutions, including liquid biopsy technologies that focus on improving cancer detection and monitoring.Roche:
Roche is committed to advancing personalized healthcare and offers a comprehensive portfolio of liquid biopsy products for accurate diagnosis and monitoring.We're grateful to work with incredible clients.









FAQs
What is the market size of breast cancer liquid biopsy?
The breast cancer liquid biopsy market is valued at approximately $3.8 billion in 2023, with a projected compound annual growth rate (CAGR) of 7.8%, indicating strong growth into the next decade.
What are the key market players or companies in the breast cancer liquid biopsy industry?
Key players in the breast cancer liquid biopsy market include Guardant Health, Exact Sciences Corporation, and Biocept. These companies remain at the forefront, continually innovating diagnostic solutions and enhancing patient outcomes.
What are the primary factors driving the growth in the breast cancer liquid biopsy industry?
Growth in the breast cancer liquid biopsy market is driven by increasing cancer prevalence, advancements in technology, and a shift towards minimally invasive diagnostics. Additionally, rising public awareness and funding for cancer research contribute significantly.
Which region is the fastest Growing in the breast cancer liquid biopsy market?
Asia Pacific is expected to be the fastest-growing region in the breast cancer liquid biopsy market, increasing from $0.83 billion in 2023 to $1.79 billion by 2033, driven by rising healthcare investments and cancer awareness initiatives.
Does ConsaInsights provide customized market report data for the breast cancer liquid biopsy industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the breast cancer liquid biopsy industry, ensuring clients receive insights that align with their business objectives and strategies.
What deliverables can I expect from this breast cancer liquid biopsy market research project?
Deliverables include comprehensive reports detailing market size, growth forecasts, competitive analysis, key trends, regional insights, and segmentation data. Clients will receive actionable insights specifically tailored to breast cancer liquid biopsy.
What are the market trends of breast cancer liquid biopsy?
Key trends in the breast cancer liquid biopsy market include increased adoption of liquid biopsy technologies, focus on early detection, and integration of artificial intelligence for data analysis. Notably, growing preferences for personalized medicine are reshaping market dynamics.