C Reactive Protein Testing Market Report
Published Date: 31 January 2026 | Report Code: c-reactive-protein-testing
C Reactive Protein Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the C Reactive Protein (CRP) Testing market from 2023 to 2033, including insights on market size, growth trends, segment analysis, regional performance, and key players shaping the industry landscape.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
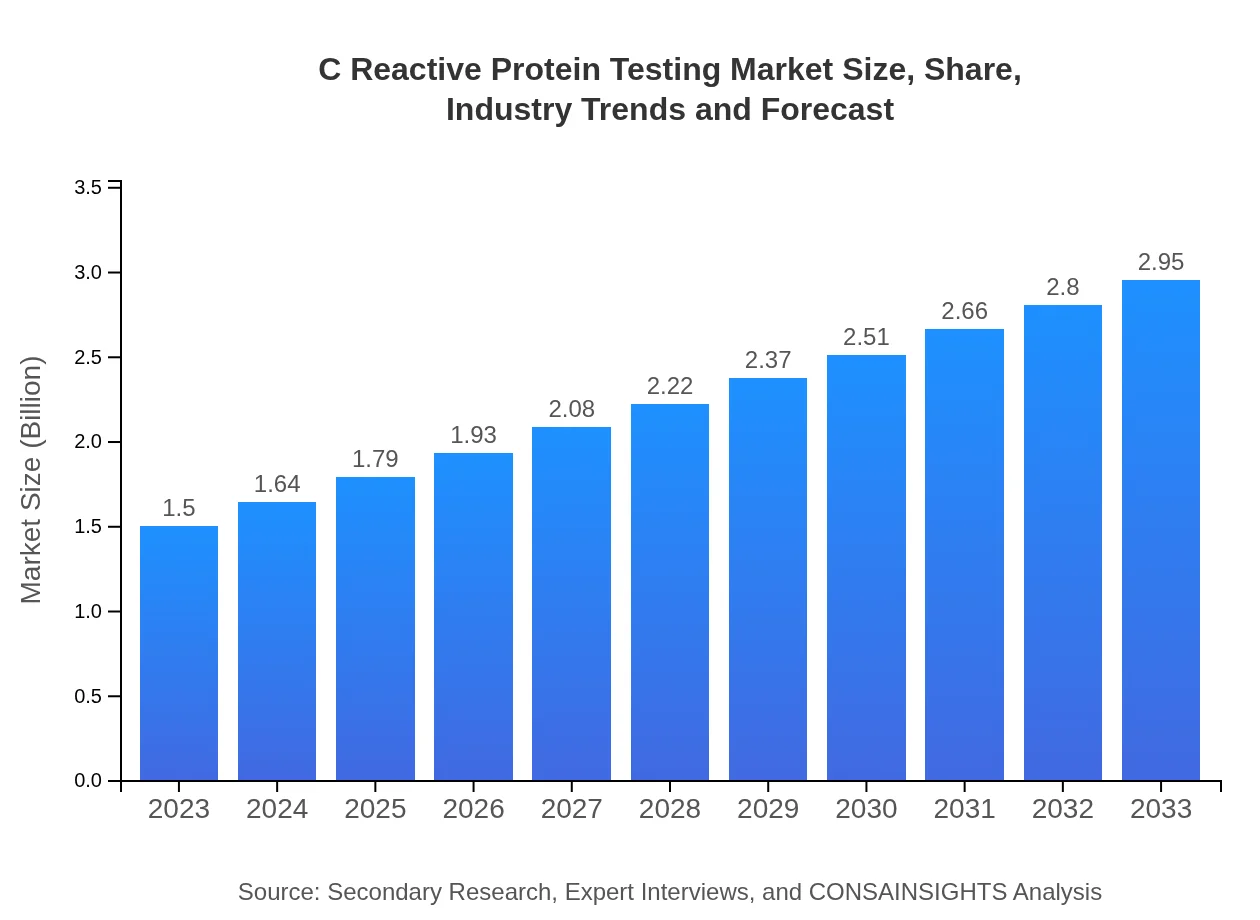
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $2.95 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Thermo Fisher Scientific, Beckman Coulter |
| Last Modified Date | 31 January 2026 |
C Reactive Protein Testing Market Overview
Customize C Reactive Protein Testing Market Report market research report
- ✔ Get in-depth analysis of C Reactive Protein Testing market size, growth, and forecasts.
- ✔ Understand C Reactive Protein Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in C Reactive Protein Testing
What is the Market Size & CAGR of C Reactive Protein Testing market in 2023?
C Reactive Protein Testing Industry Analysis
C Reactive Protein Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
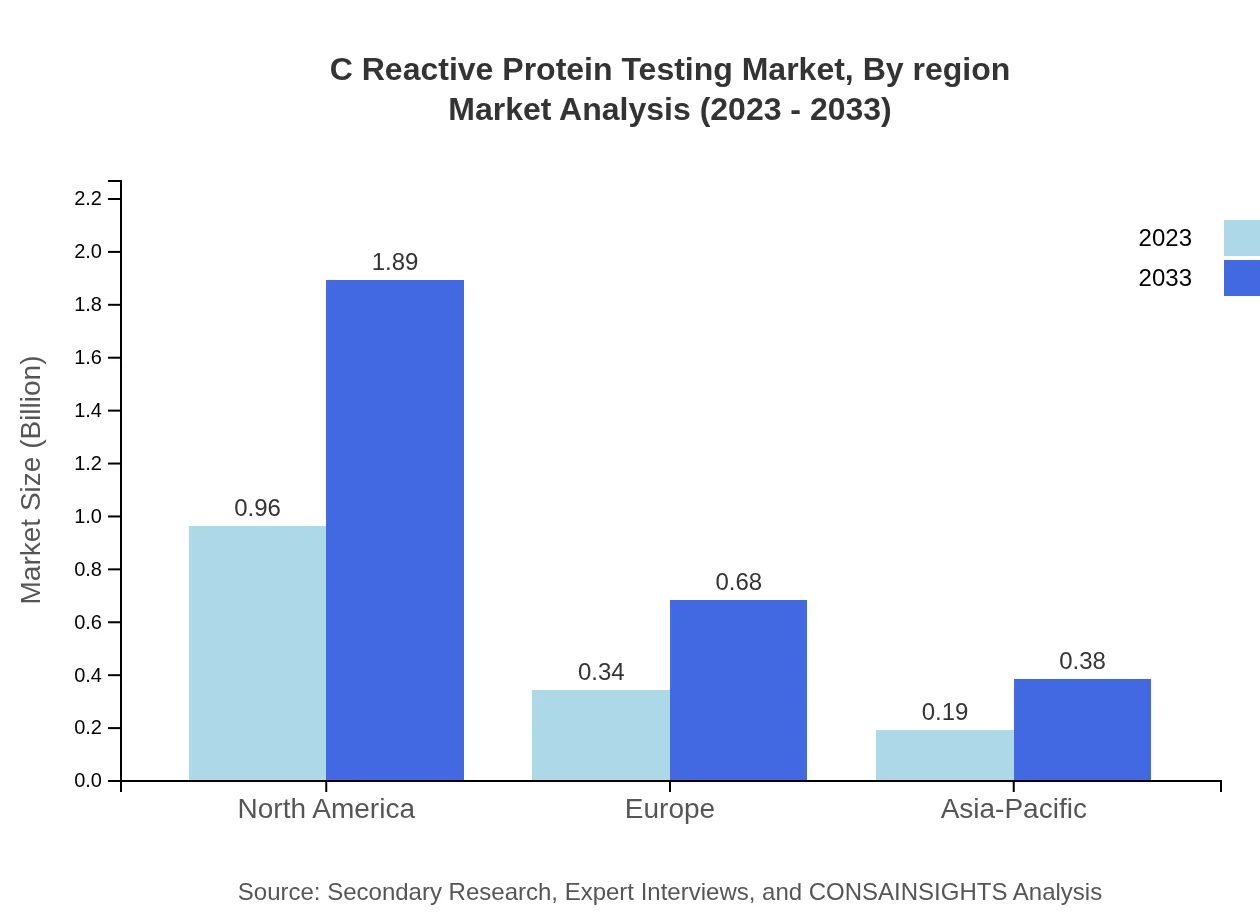
C Reactive Protein Testing Market Analysis Report by Region
Europe C Reactive Protein Testing Market Report:
The European market for C Reactive Protein Testing is expected to grow from $0.37 billion in 2023 to $0.72 billion by 2033. The region benefits from stringent healthcare regulations, increasing demand for diagnostic tests, and significant investments in research and technology.Asia Pacific C Reactive Protein Testing Market Report:
The Asia Pacific region is witnessing significant growth, with the market expected to reach $0.62 billion by 2033, up from $0.31 billion in 2023. This growth is driven by an increasing prevalence of lifestyle diseases, enhancements in healthcare infrastructure, and rising healthcare spending.North America C Reactive Protein Testing Market Report:
North America holds a dominant position in the market, projected to grow from $0.55 billion in 2023 to $1.07 billion by 2033. The region's growth is fueled by advanced healthcare infrastructure, widespread adoption of diagnostic testing, and rising awareness about the importance of early disease detection.South America C Reactive Protein Testing Market Report:
In South America, the market is anticipated to grow from $0.12 billion in 2023 to $0.23 billion by 2033. Factors contributing to this growth include an increasing focus on chronic disease management and the expansion of healthcare access.Middle East & Africa C Reactive Protein Testing Market Report:
The Middle East and Africa region is also set for growth, with the market projected to increase from $0.16 billion in 2023 to $0.31 billion by 2033. This growth is attributed to improving healthcare facilities and diagnostic capabilities across several countries.Tell us your focus area and get a customized research report.
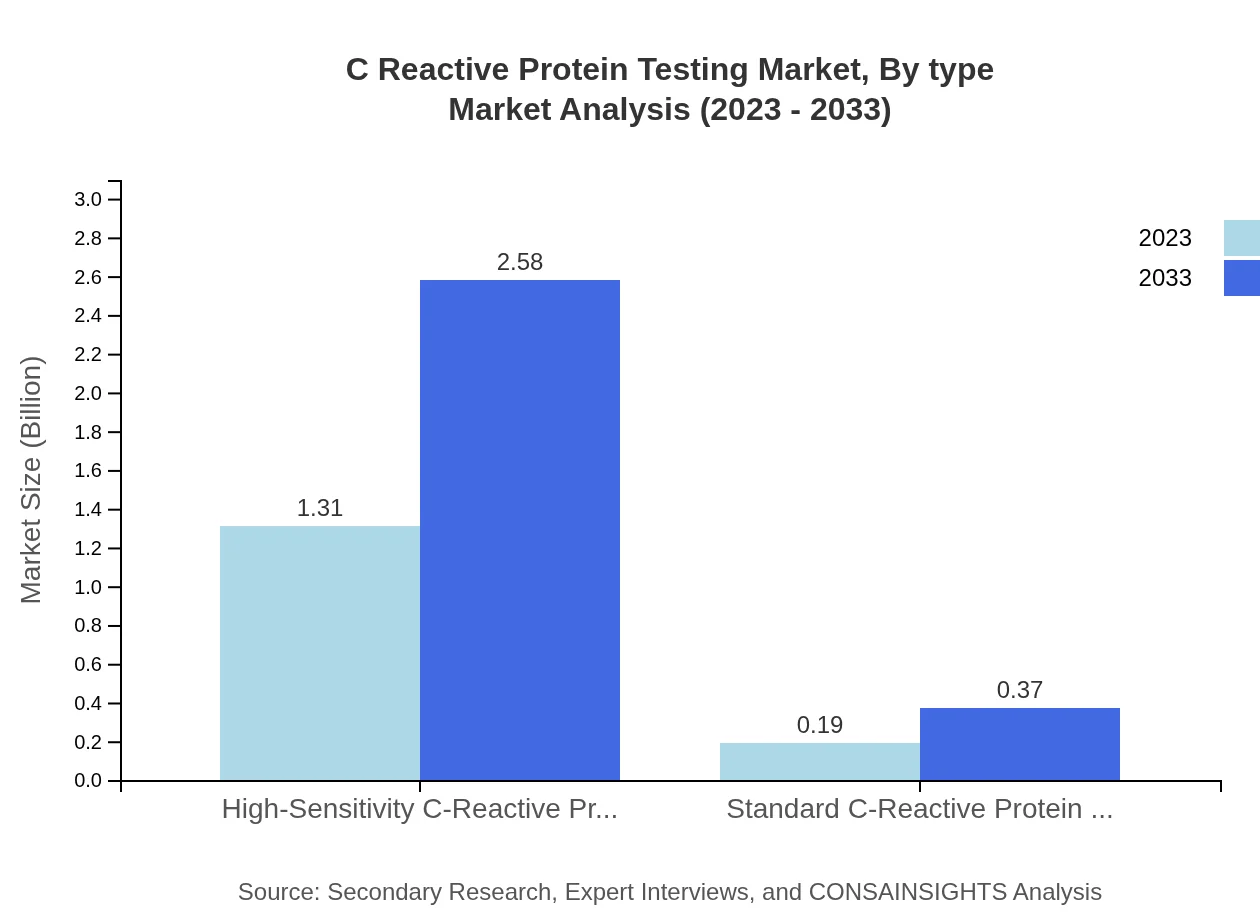
C Reactive Protein Testing Market Analysis By Type
The market segmentation by type comprises High-Sensitivity CRP Test and Standard CRP Test. The High-Sensitivity CRP Test dominates with a market share of 87.53% in 2023 and is expected to grow from $1.31 billion to $2.58 billion by 2033. The Standard CRP Test holds 12.47% market share, anticipated to rise from $0.19 billion to $0.37 billion over the same period.
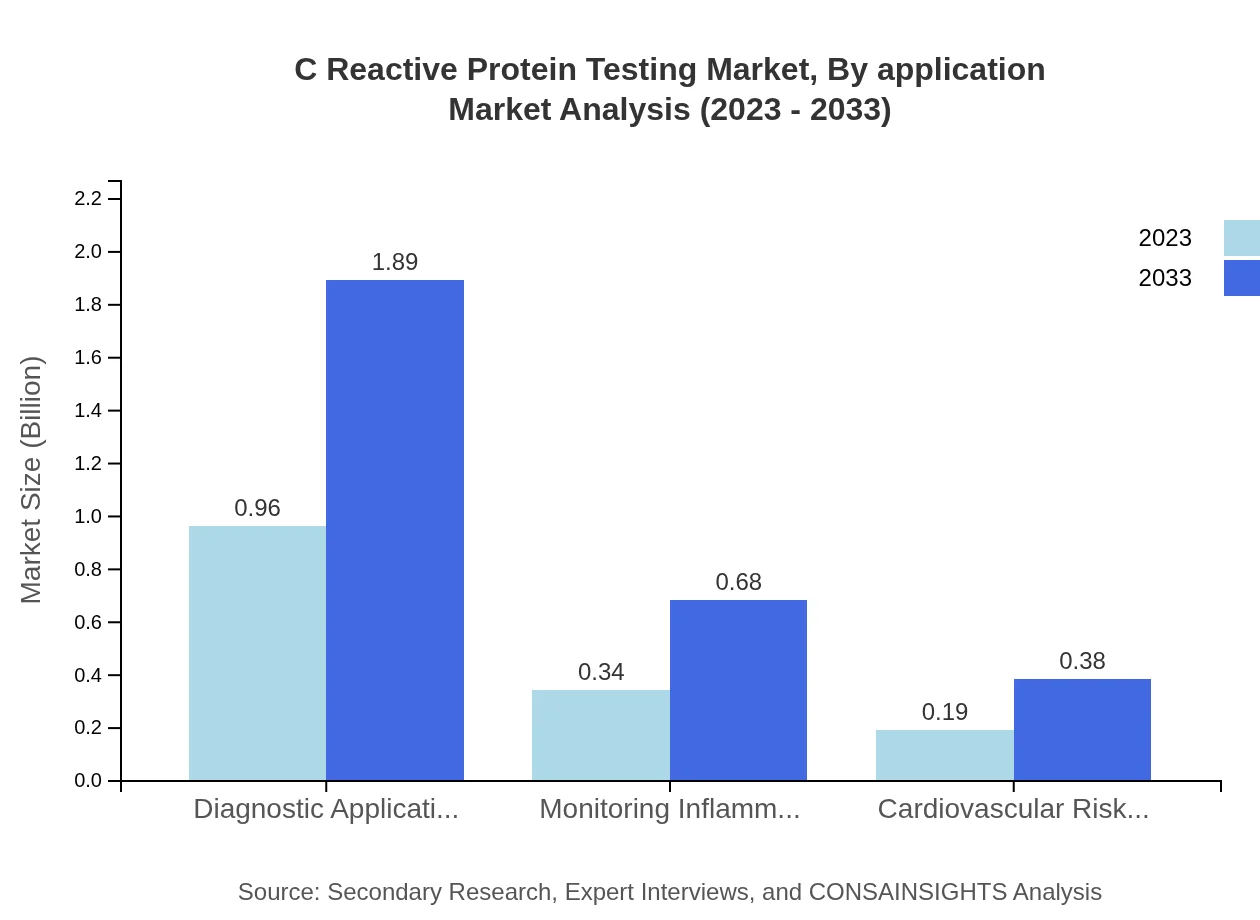
C Reactive Protein Testing Market Analysis By Application
The market by application is categorized into Diagnostic Applications, Monitoring Inflammatory Diseases, and Cardiovascular Risk Assessment. Diagnostic Applications hold a substantial market share of 64.1% in 2023, growing from $0.96 billion to $1.89 billion by 2033. Monitoring Inflammatory Diseases represents 22.95% and is projected to increase from $0.34 billion to $0.68 billion, while Cardiovascular Risk Assessment accounts for 12.95% of the market, expected to rise from $0.19 billion to $0.38 billion.
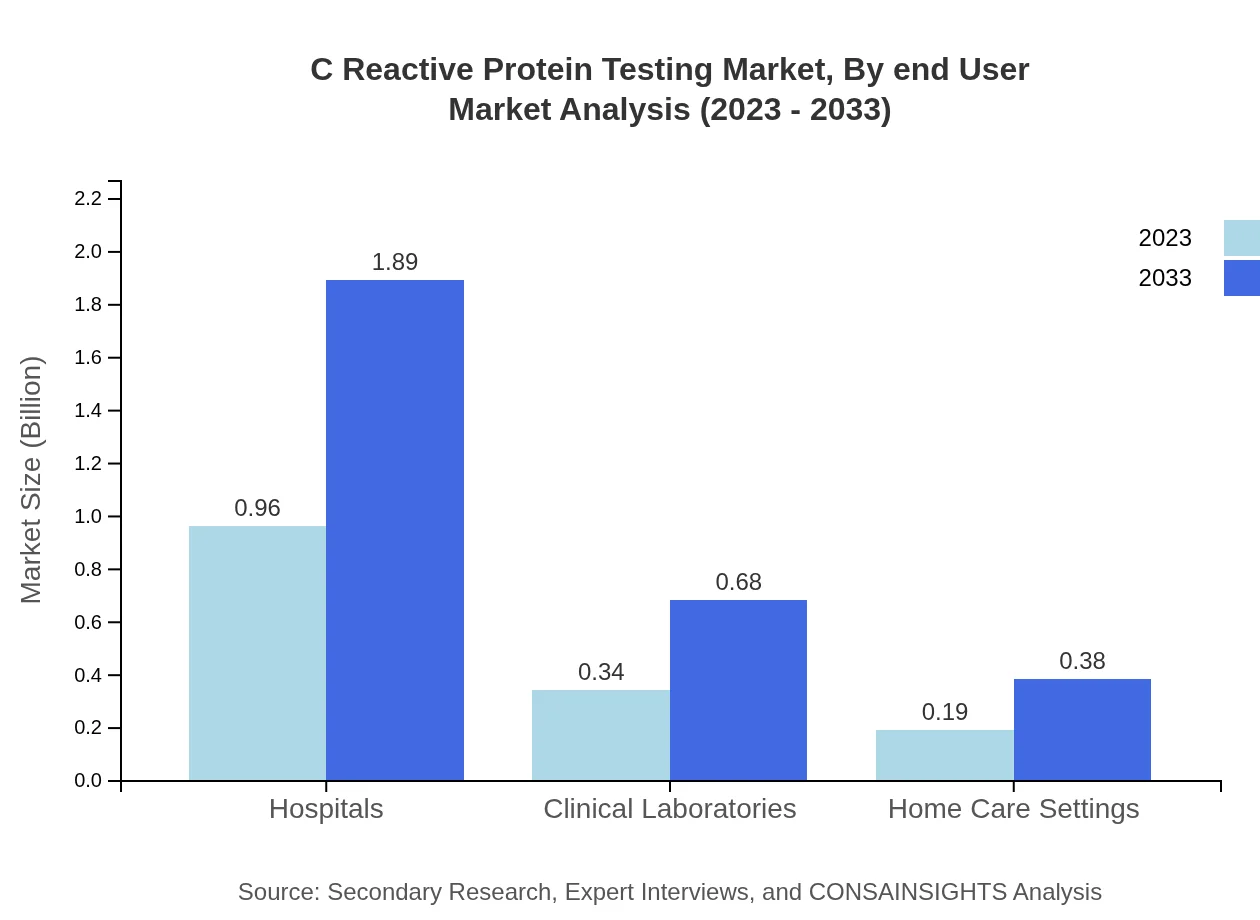
C Reactive Protein Testing Market Analysis By End User
The end-user segment includes Hospitals, Clinical Laboratories, and Home Care Settings. Hospitals dominate with a market share of 64.1%, growing from $0.96 billion to $1.89 billion by 2033. Clinical Laboratories will expand from $0.34 billion to $0.68 billion, maintaining a share of 22.95%. Home Care Settings account for 12.95% and are expected to grow from $0.19 billion to $0.38 billion.
C Reactive Protein Testing Market Analysis By Region
The regional analysis indicates North America leads the market (64.1%), followed by Europe (22.95%) and Asia-Pacific (12.95%). Each region displays unique growth drivers, including healthcare access, technological advancements, and disease prevalence.
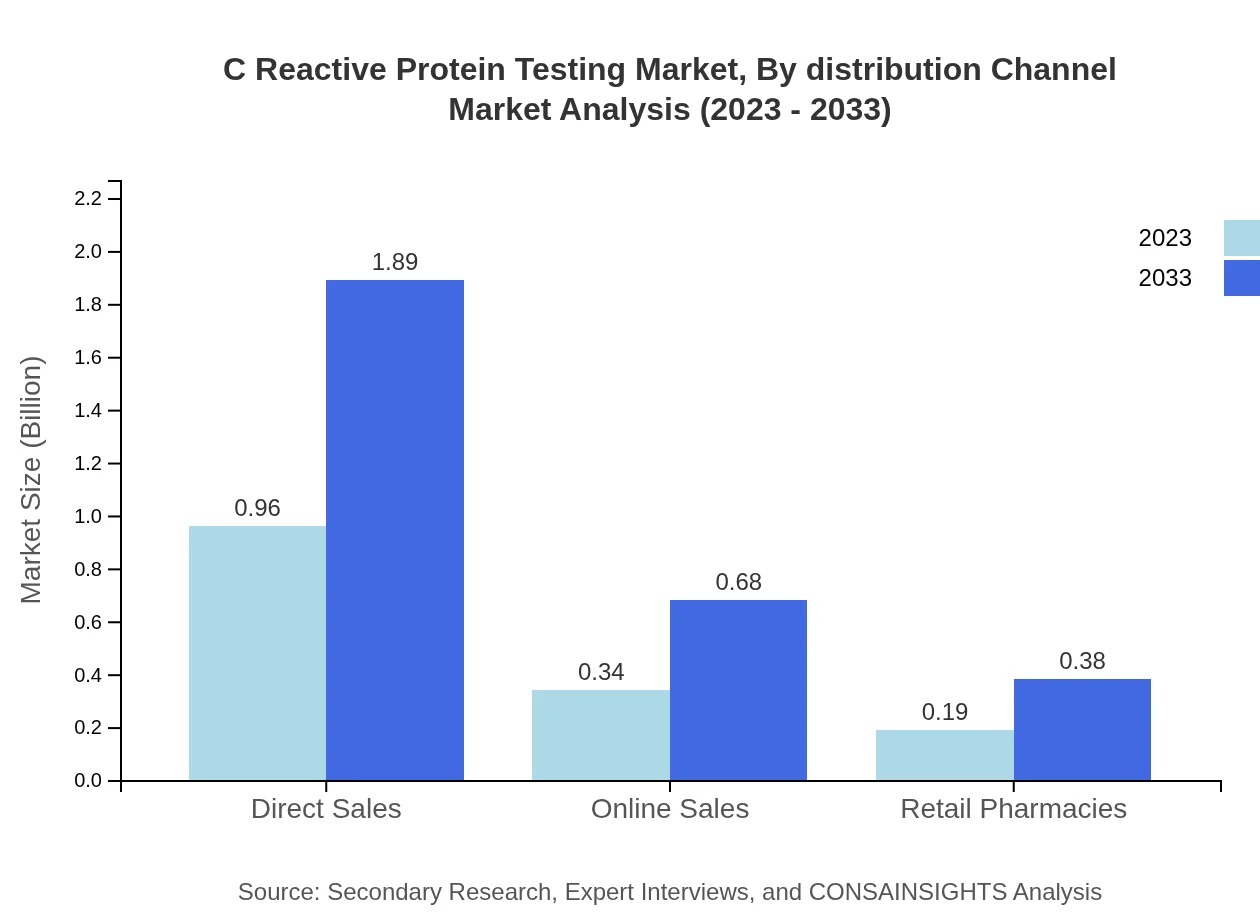
C Reactive Protein Testing Market Analysis By Distribution Channel
Distribution channels comprise Direct Sales, Online Sales, and Retail Pharmacies. Direct Sales account for 64.1% of the market, growing from $0.96 billion to $1.89 billion by 2033. Online Sales hold a share of 22.95%, projected to rise from $0.34 billion to $0.68 billion, while Retail Pharmacies possess a 12.95% share, expected to grow from $0.19 billion to $0.38 billion.
C Reactive Protein Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in C Reactive Protein Testing Industry
Roche Diagnostics:
Roche Diagnostics is a pioneer in medical diagnostics, offering innovative CRP testing solutions that enhance clinical decision-making and patient care.Abbott Laboratories:
Abbott Laboratories is a global healthcare company known for its extensive range of diagnostic tools, including advanced CRP assay systems that provide rapid results.Siemens Healthineers:
Siemens Healthineers provides cutting-edge CRP testing equipment and solutions, setting benchmarks for quality and efficiency in diagnostics.Thermo Fisher Scientific:
Thermo Fisher Scientific is renowned for its contributions to laboratory and diagnostic technologies, including innovative CRP testing platforms.Beckman Coulter:
Beckman Coulter specializes in laboratory instrumentation and assays, offering reliable CRP testing solutions that improve patient outcomes.We're grateful to work with incredible clients.









FAQs
What is the market size of c Reactive Protein Testing?
The global C-Reactive Protein Testing market is valued at approximately $1.5 billion as of 2023, with a robust CAGR of 6.8%. This growth is anticipated to reflect an expanding demand for diagnostic testing and increasing awareness of inflammatory diseases.
What are the key market players or companies in this c Reactive Protein Testing industry?
Prominent market players in the C-Reactive Protein Testing industry include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific. These companies lead through innovation and extensive product portfolios in clinical diagnostics and testing.
What are the primary factors driving the growth in the c Reactive Protein Testing industry?
Key growth factors include the rising prevalence of chronic inflammatory diseases, increasing healthcare expenditure, advancements in diagnostic technology, and growing awareness of preventive healthcare. Additionally, the rise in aging populations is also fueling market demand.
Which region is the fastest Growing in the c Reactive Protein Testing?
Among the regions analyzed, North America is the fastest-growing market for C-Reactive Protein Testing, projected to grow from $0.55 billion in 2023 to $1.07 billion by 2033, reflecting a strong healthcare infrastructure and high demand for diagnostic tools.
Does ConsaInsights provide customized market report data for the c Reactive Protein Testing industry?
Yes, ConsaInsights offers customized market report data tailored to the C-Reactive Protein Testing industry. Our reports can be adjusted to focus on specific regions, market segments, or emergent trends to better meet your research needs.
What deliverables can I expect from this c Reactive Protein Testing market research project?
Deliverables from the C-Reactive Protein Testing market research project typically include comprehensive market analysis, trends and insights reports, competitive landscape overviews, regional and segment data, along with actionable recommendations for market entry or expansion.
What are the market trends of c Reactive Protein Testing?
Current trends in the C-Reactive Protein Testing market include the increasing adoption of high-sensitivity tests, growth in home care testing settings, collaborations among key players for innovation, and a shift towards online sales channels for broader accessibility.