Cancer Biomarkers Market Report
Published Date: 31 January 2026 | Report Code: cancer-biomarkers
Cancer Biomarkers Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cancer Biomarkers market from 2023 to 2033, offering insights into market size, growth trends, technological advancements, regional performance, and key industry players.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
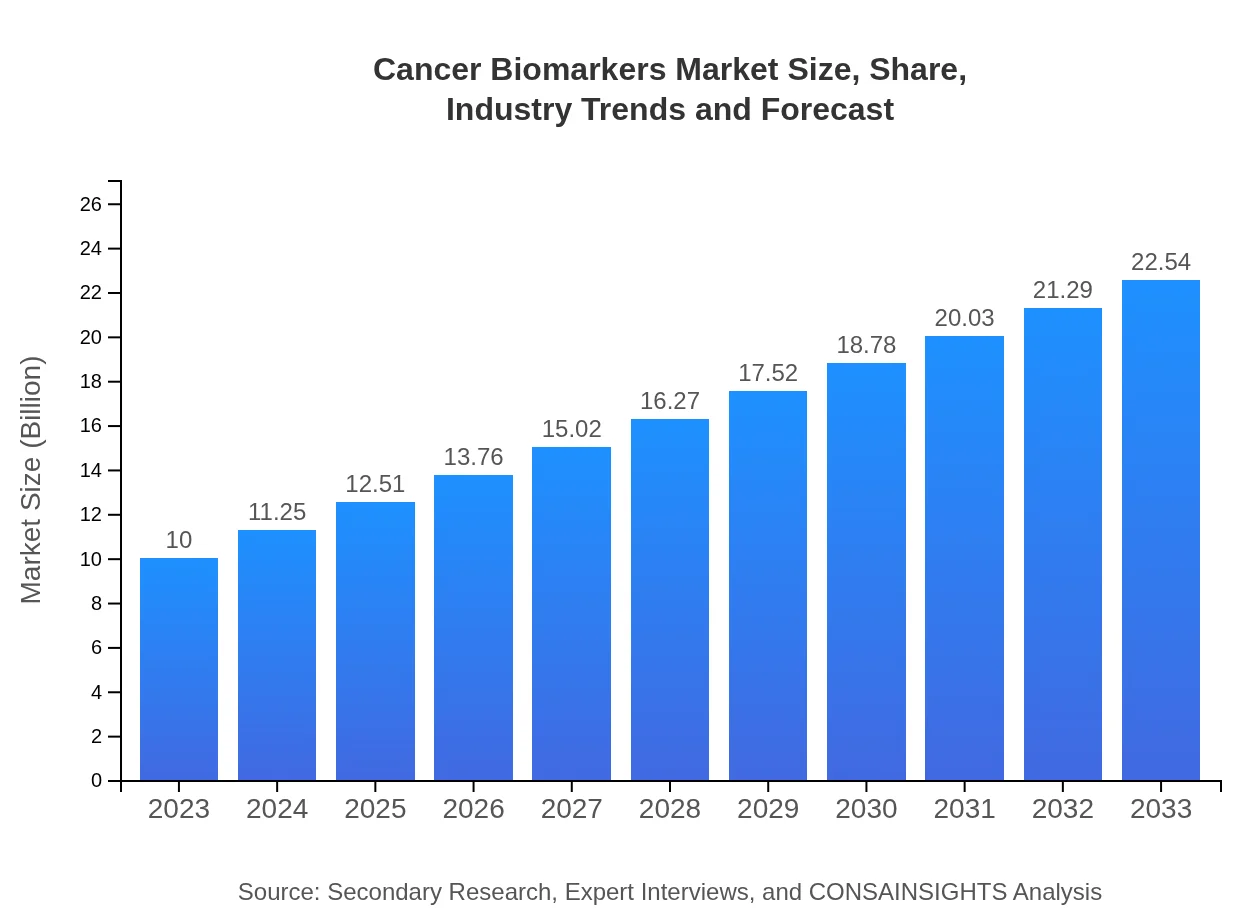
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 8.2% |
| 2033 Market Size | $22.54 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Illumina, Inc. |
| Last Modified Date | 31 January 2026 |
Cancer Biomarkers Market Overview
Customize Cancer Biomarkers Market Report market research report
- ✔ Get in-depth analysis of Cancer Biomarkers market size, growth, and forecasts.
- ✔ Understand Cancer Biomarkers's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cancer Biomarkers
What is the Market Size & CAGR of Cancer Biomarkers market in 2023 and 2033?
Cancer Biomarkers Industry Analysis
Cancer Biomarkers Market Segmentation and Scope
Tell us your focus area and get a customized research report.
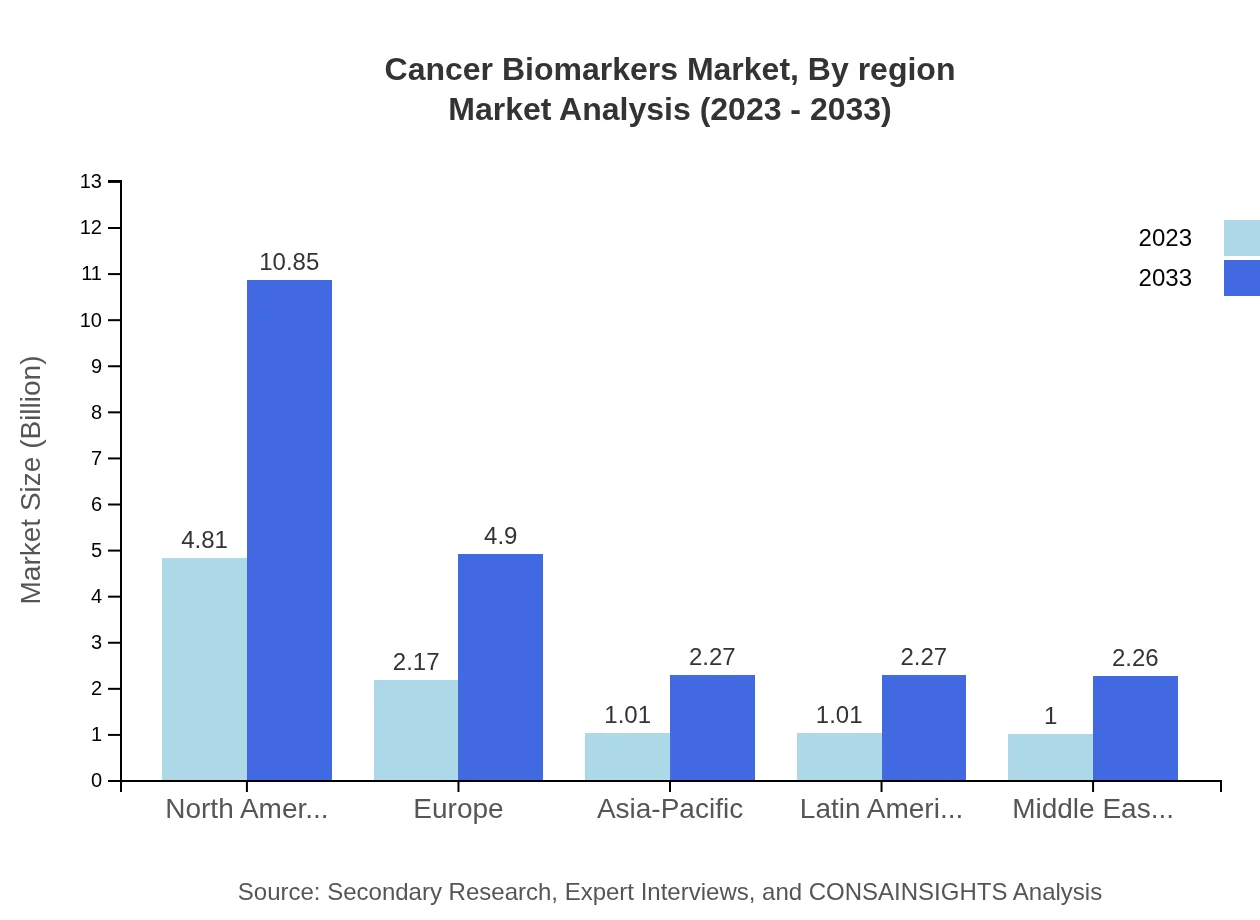
Cancer Biomarkers Market Analysis Report by Region
Europe Cancer Biomarkers Market Report:
In Europe, the Cancer Biomarkers market is expected to increase from USD 2.48 billion in 2023 to USD 5.59 billion by 2033. The region benefits from a well-established healthcare system, increasing demand for early cancer diagnosis, and a rising focus on research and innovation. Government initiatives promoting cancer research and funding programs will bolster market growth.Asia Pacific Cancer Biomarkers Market Report:
In the Asia Pacific region, the Cancer Biomarkers market is expected to grow from USD 2.00 billion in 2023 to USD 4.52 billion by 2033. Factors such as a growing patient population, increased healthcare expenditure, and rising awareness of cancer diagnostics significantly boost market growth. Partnerships among key players and governments to enhance cancer care will also play a critical role in this expansion.North America Cancer Biomarkers Market Report:
North America is poised to maintain its dominance in the Cancer Biomarkers market, with a size increase from USD 3.51 billion in 2023 to USD 7.91 billion by 2033. This growth is fueled by the presence of advanced healthcare facilities, a high rate of research activities, and significant funding directed towards cancer research. The robust adoption of personalized medicine and advancements in diagnostic technologies further drive this market.South America Cancer Biomarkers Market Report:
The Latin America market is projected to grow from USD 0.68 billion in 2023 to USD 1.54 billion by 2033, supported by increasing investments in healthcare infrastructure and rising cancer prevalence. Awareness programs and government initiatives to improve cancer screening and treatment access will contribute to market expansion in this region.Middle East & Africa Cancer Biomarkers Market Report:
The Middle East and Africa market is anticipated to expand from USD 1.32 billion in 2023 to USD 2.98 billion by 2033. The growth is supported by increasing healthcare expenditures, gradual improvements in healthcare infrastructure, and initiatives aimed at enhancing public health awareness regarding cancer screening and diagnosis.Tell us your focus area and get a customized research report.
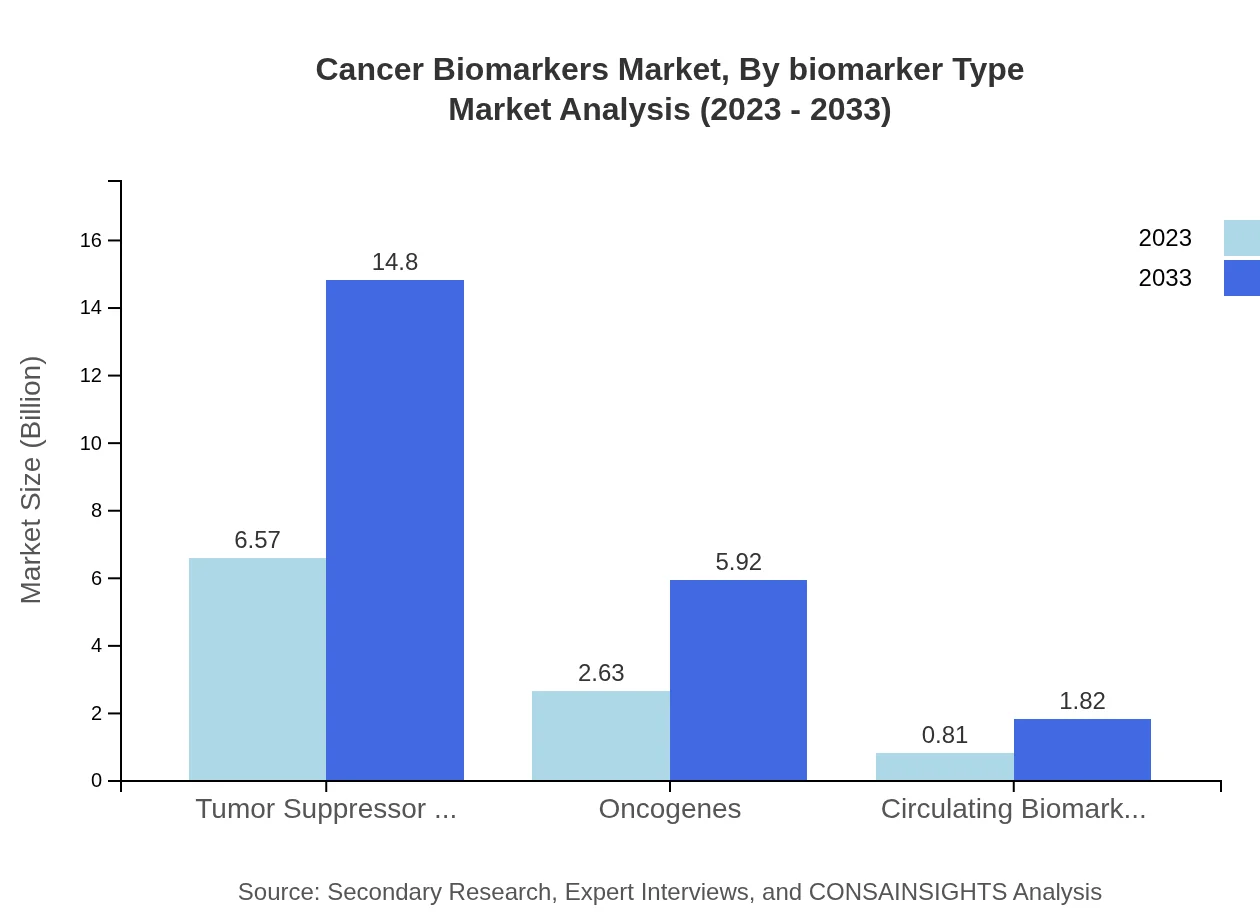
Cancer Biomarkers Market Analysis By Biomarker Type
The Cancer Biomarkers market, categorized by biomarker type, sees significant contributions from tumor suppressor genes, with a market size growing from USD 6.57 billion in 2023 to USD 14.80 billion by 2033, representing a 65.66% share in the market. Oncogenes and circulating biomarkers follow, contributing to early cancer detection and personalized treatments.
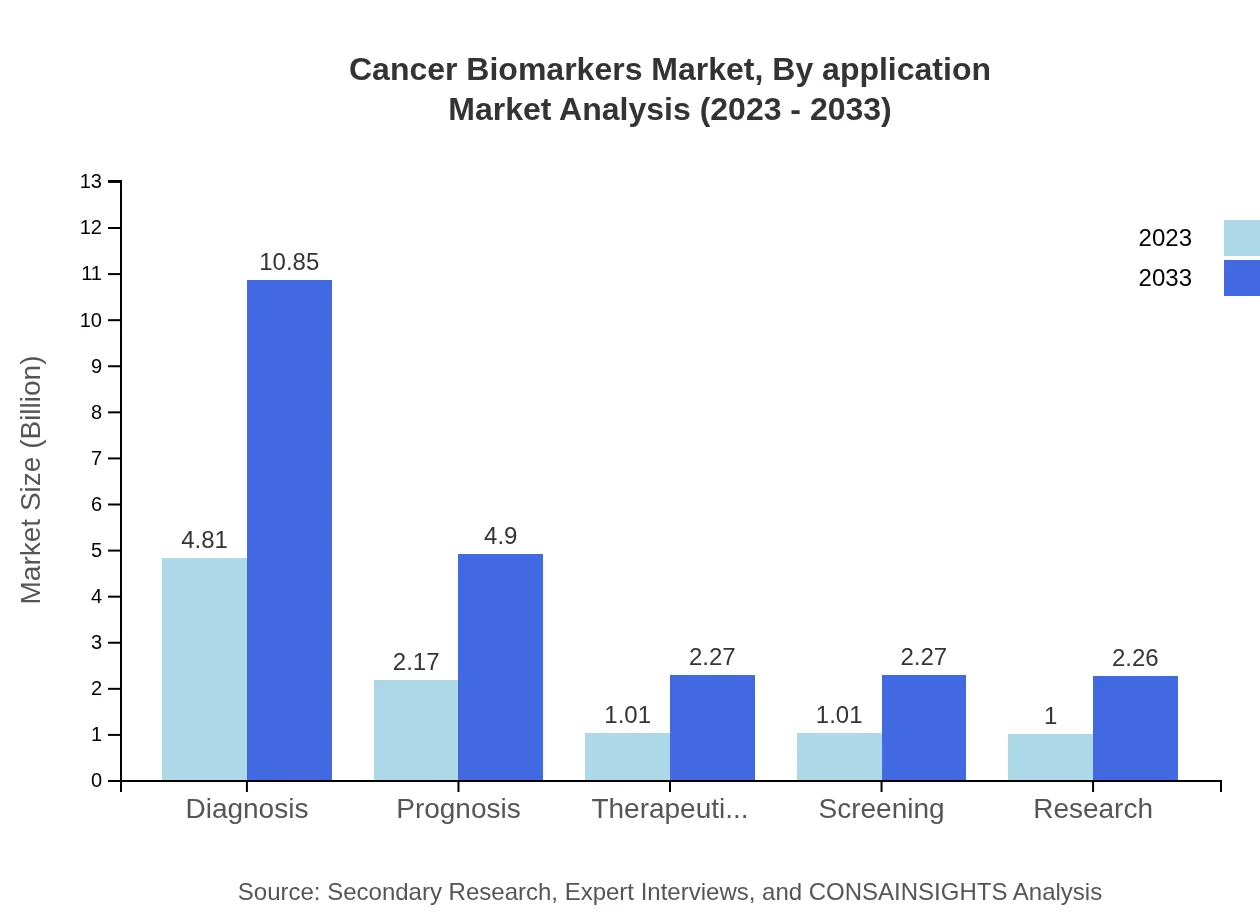
Cancer Biomarkers Market Analysis By Application
The application segmentation shows that the diagnosis of cancer accounts for 48.13% market share, significantly increasing from USD 4.81 billion in 2023 to USD 10.85 billion by 2033. Prognostic applications and therapeutic monitoring are also noteworthy, indicating a strong focus on comprehensive cancer management through effective biomarker utilization.
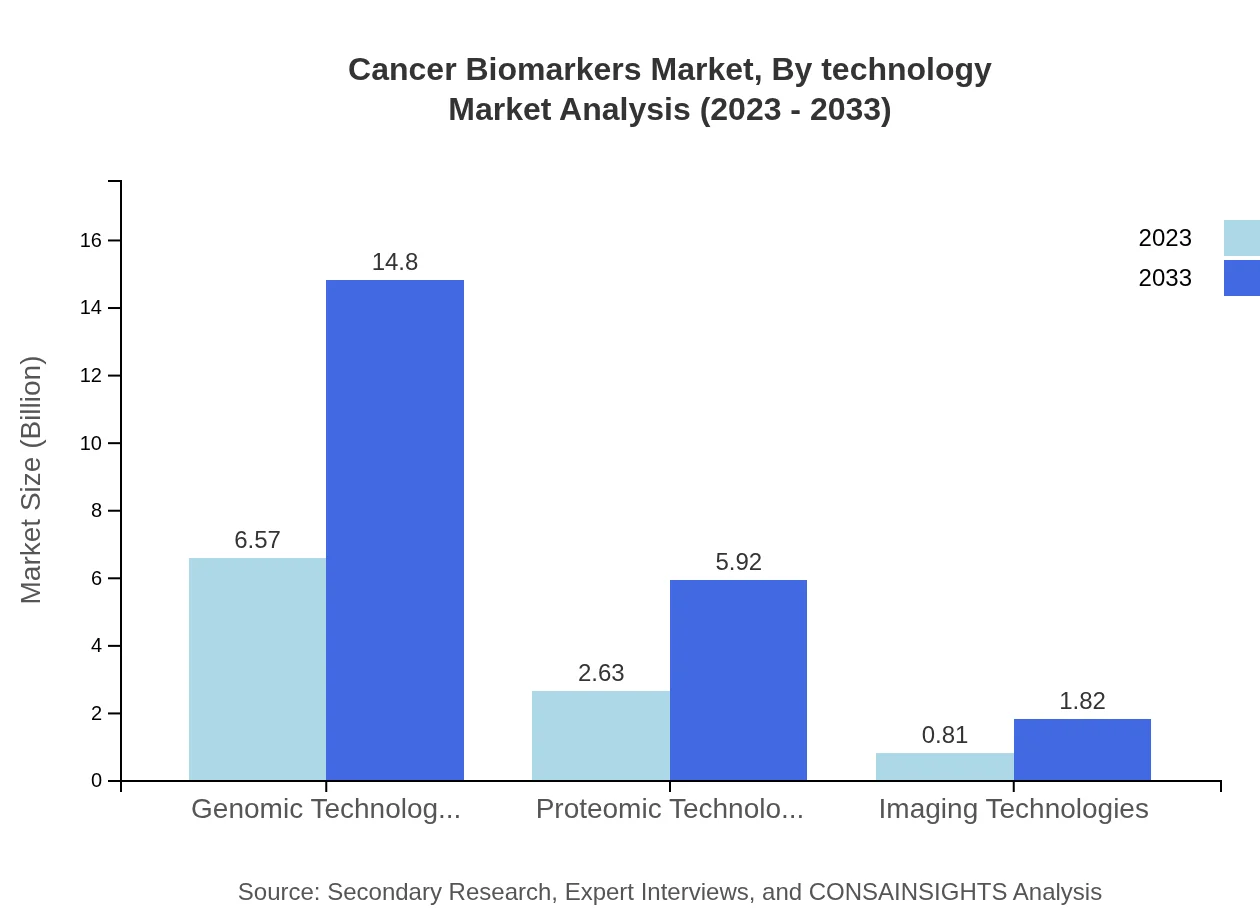
Cancer Biomarkers Market Analysis By Technology
The market demonstrates a dominant trend in genomic technologies, representing 65.66% of the market size in 2023, projected to grow from USD 6.57 billion to USD 14.80 billion by 2033. Proteomic technologies follow at 26.27% share, showcasing the vital role of molecular profiling in cancer diagnostics and treatment.
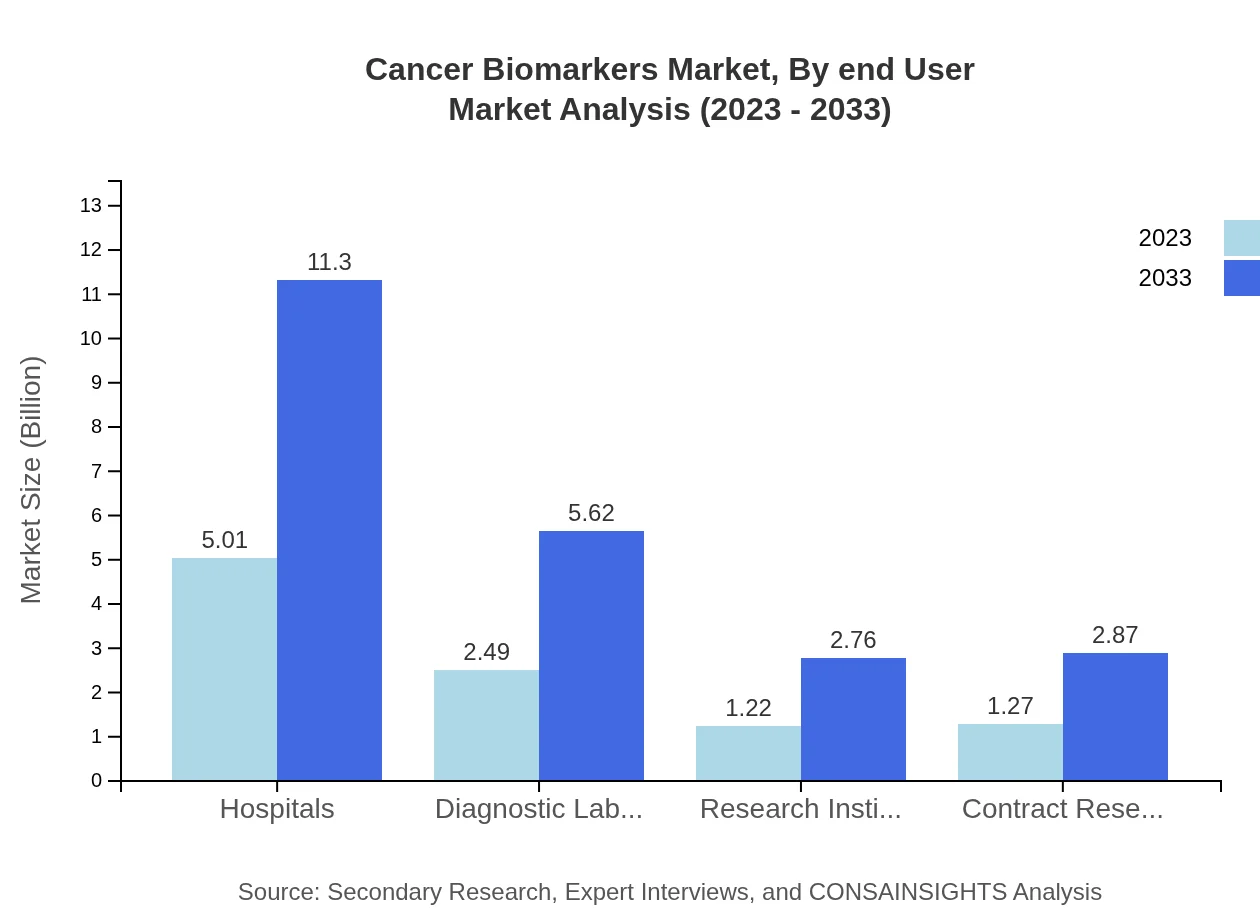
Cancer Biomarkers Market Analysis By End User
Hospitals and diagnostic laboratories lead in end-user distribution, with hospitals holding a 50.14% market share. The size is projected to rise from USD 5.01 billion in 2023 to USD 11.30 billion by 2033, reflecting the central role of healthcare facilities in delivering cancer diagnostic services.
Cancer Biomarkers Market Analysis By Region
The regional analysis highlights the notable growth prospects across various areas, with North America leading, followed by Europe and the Asia Pacific. This segment emphasizes the varying degrees of market development, healthcare access, and investment in cancer research across regions.
Cancer Biomarkers Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cancer Biomarkers Industry
Roche Diagnostics:
A leader in the molecular diagnostics market, Roche Diagnostics focuses on developing innovative solutions in cancer biomarkers to improve diagnosis and treatment.Abbott Laboratories:
Abbott specializes in laboratory diagnostics and is known for its advancements in biomarker technologies that aid in cancer screening and personalized therapies.Thermo Fisher Scientific:
A leading provider of life science solutions, Thermo Fisher is pivotal in advancing genomic technologies and biomarker research for cancer.Illumina, Inc.:
Illumina empowers the genomics revolution with its sequencing and array technologies, significantly enhancing molecular diagnostics in oncology.We're grateful to work with incredible clients.









FAQs
What is the market size of cancer Biomarkers?
The global cancer biomarkers market is valued at approximately $10 billion in 2023, with a projected growth rate (CAGR) of 8.2%. This growth is expected to sustain up to 2033, driven by advancements in cancer diagnosis and treatments.
What are the key market players or companies in the cancer Biomarkers industry?
Key players in the cancer biomarkers market include Genomic Health, Roche Diagnostics, and Illumina. These companies lead in innovation and product development, significantly influencing market trends and advancements in cancer diagnostics.
What are the primary factors driving the growth in the cancer Biomarkers industry?
Growth in the cancer-biomarkers market is driven by increasing cancer prevalence, advancements in molecular biology, and demand for personalized medicine. Additionally, the rise in research activities and technological innovations in biomarker discovery significantly contribute to market expansion.
Which region is the fastest Growing in the cancer Biomarkers market?
North America is the fastest-growing region, with a market size projected to grow from $3.51 billion in 2023 to $7.91 billion by 2033. This growth is fueled by robust healthcare infrastructure and increased funding for cancer research initiatives.
Does ConsaInsights provide customized market report data for the cancer Biomarkers industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the cancer-biomarkers industry. This customization helps businesses gain critical insights that facilitate strategic decision-making in this competitive arena.
What deliverables can I expect from this cancer Biomarkers market research project?
Deliverables from the cancer-biomarkers market research include comprehensive market analysis reports, competitive landscape assessments, segment insights, and trend forecasts to aid in informed decision-making and strategic planning for stakeholders.
What are the market trends of cancer Biomarkers?
Current trends in the cancer-biomarkers market include a shift towards liquid biopsies, an increase in partnerships for biomarker development, and a growing emphasis on targeted therapies. Innovations in genomic and proteomic technologies are also prevalent.