Cancer Biopsies Market Report
Published Date: 31 January 2026 | Report Code: cancer-biopsies
Cancer Biopsies Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cancer Biopsies market, focusing on market size, growth potential, key regional insights, and future trends from 2023 to 2033. It aims to deliver valuable insights for stakeholders and industry players to make informed decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
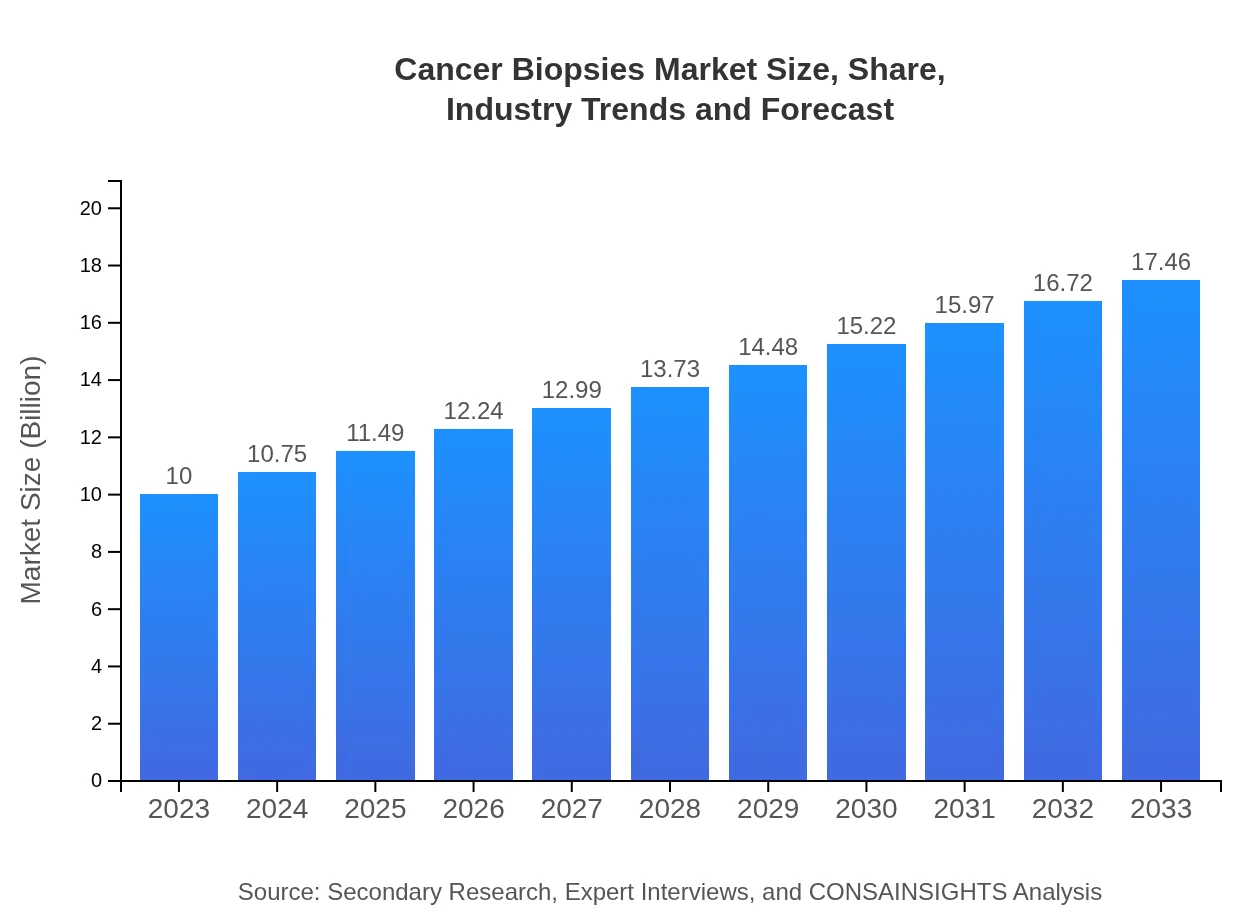
| 2023 Market Size | $10.00 Billion |
| CAGR (2023-2033) | 5.6% |
| 2033 Market Size | $17.46 Billion |
| Top Companies | Roche Diagnostics, Thermo Fisher Scientific, Becton, Dickinson and Company (BD), Illumina, Inc., Siemens Healthineers |
| Last Modified Date | 31 January 2026 |
Cancer Biopsies Market Overview
Customize Cancer Biopsies Market Report market research report
- ✔ Get in-depth analysis of Cancer Biopsies market size, growth, and forecasts.
- ✔ Understand Cancer Biopsies's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cancer Biopsies
What is the Market Size & CAGR of Cancer Biopsies market in 2023?
Cancer Biopsies Industry Analysis
Cancer Biopsies Market Segmentation and Scope
Tell us your focus area and get a customized research report.
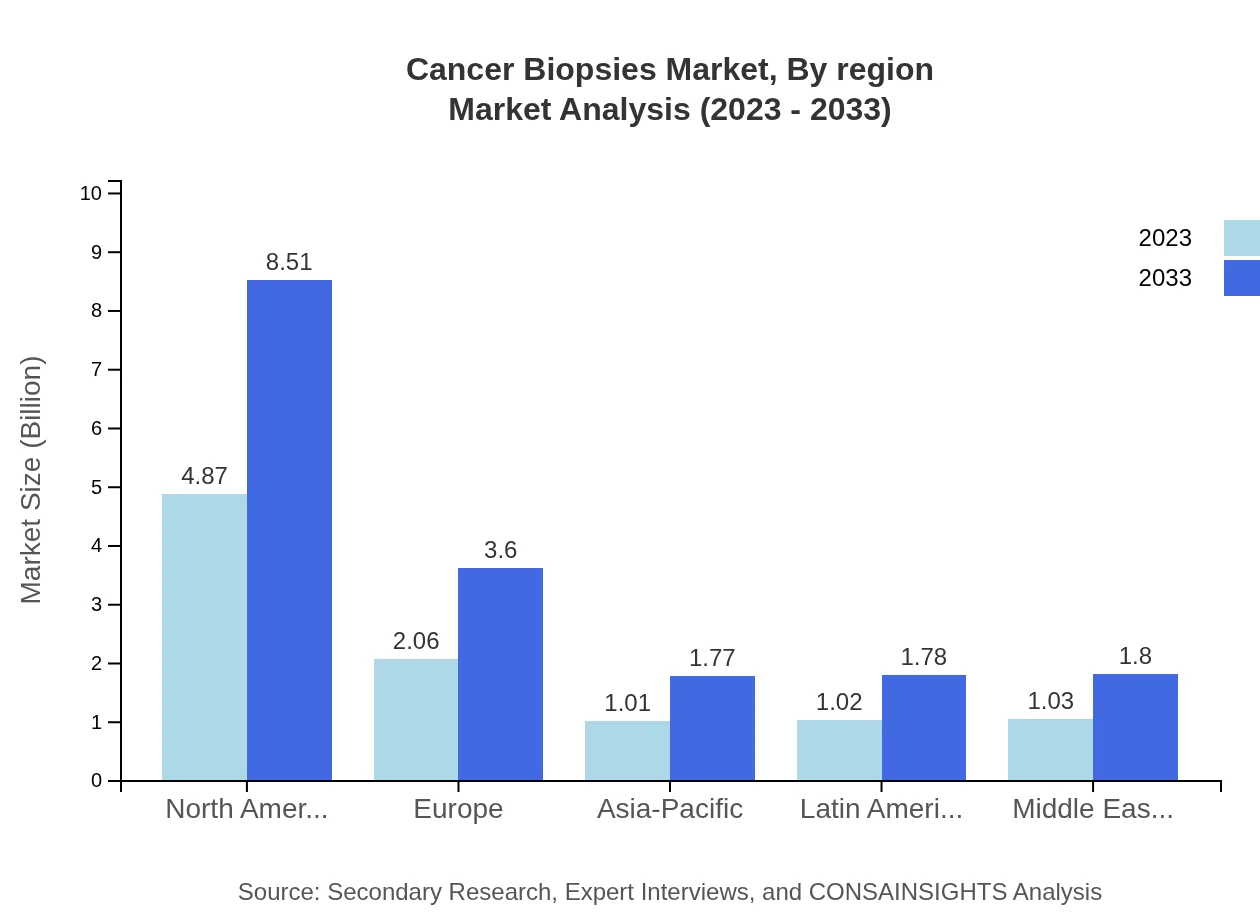
Cancer Biopsies Market Analysis Report by Region
Europe Cancer Biopsies Market Report:
The European Cancer Biopsies market will grow from $2.96 billion in 2023 to approximately $5.18 billion by 2033. The region benefits from robust healthcare policies and significant investments in cancer research.Asia Pacific Cancer Biopsies Market Report:
The Asia Pacific Cancer Biopsies market is poised to grow from $1.91 billion in 2023 to $3.34 billion in 2033, reflecting an increased focus on healthcare infrastructure and access to advanced diagnostic technologies.North America Cancer Biopsies Market Report:
North America remains the largest market for Cancer Biopsies, with a size of approximately $3.24 billion in 2023 expected to grow to $5.65 billion by 2033, due to advanced healthcare systems and high cancer prevalence rates.South America Cancer Biopsies Market Report:
The South America region is expected to expand from $0.62 billion in 2023 to $1.08 billion by 2033, driven by rising healthcare investments and awareness regarding cancer diagnosis.Middle East & Africa Cancer Biopsies Market Report:
The Middle East and Africa market is set to increase from $1.27 billion in 2023 to about $2.21 billion in 2033, driven by improved healthcare access and technological adoption in diagnostics.Tell us your focus area and get a customized research report.
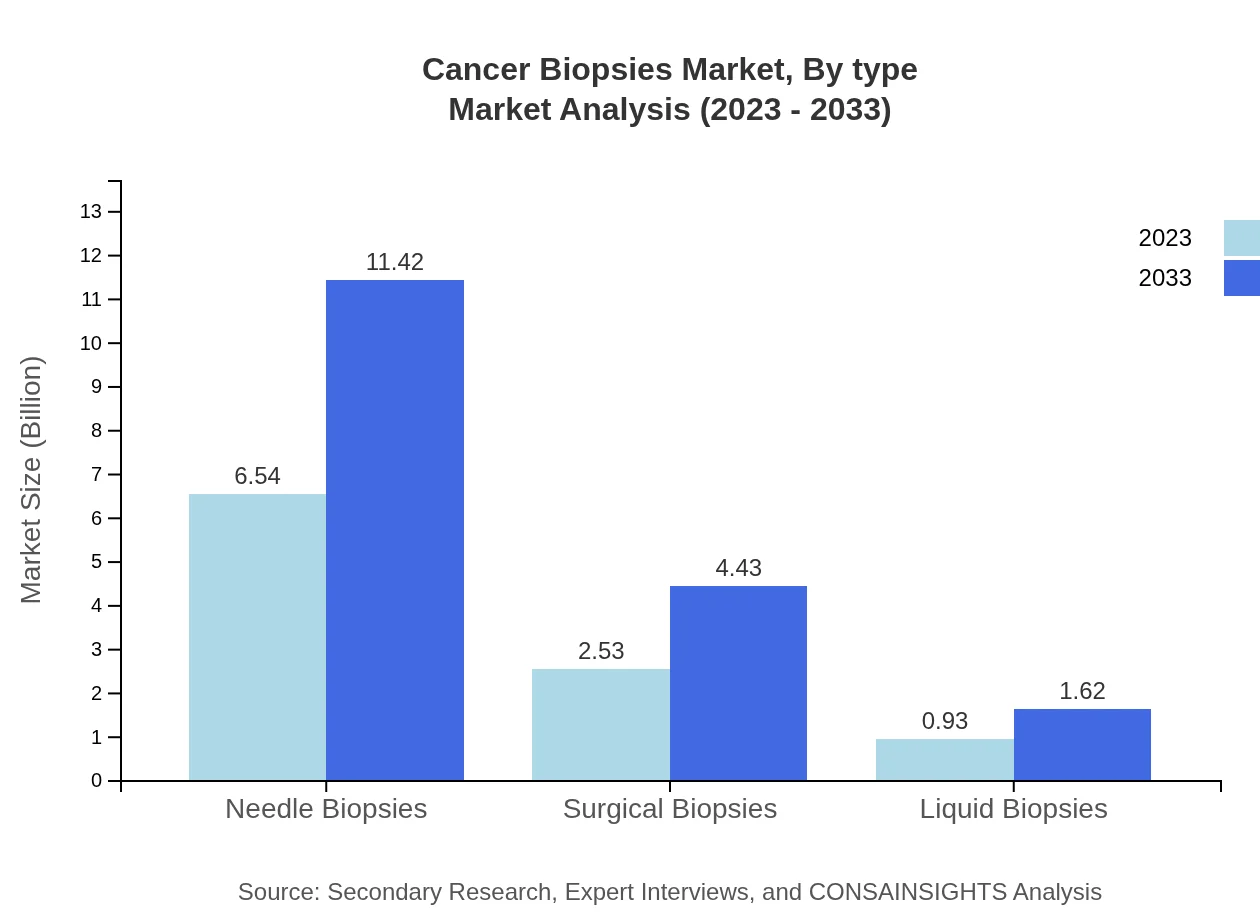
Cancer Biopsies Market Analysis By Type
Needle Biopsies dominated the market in 2023 with a size of $6.54 billion and is projected to reach $11.42 billion in 2033, holding a 65.37% market share. Surgical Biopsies and Liquid Biopsies also play crucial roles, with their respective market sizes reaching $4.43 billion and $1.62 billion by 2033.
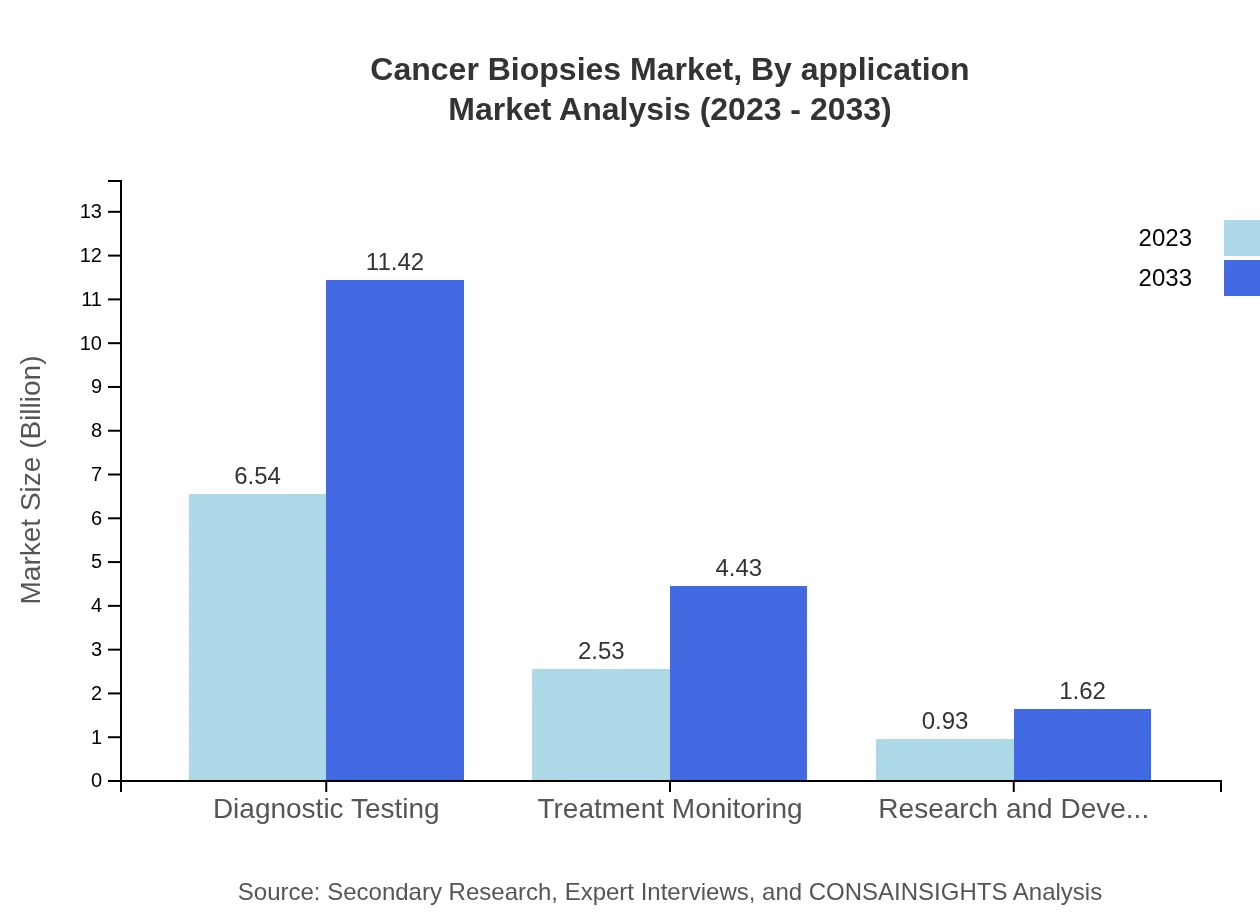
Cancer Biopsies Market Analysis By Application
In 2023, the market for Diagnostic Testing applications is expected to generate $6.54 billion, indicating a 65.37% share. As accuracy in cancer diagnosis becomes more critical, demand for Treatment Monitoring and Research and Development applications will also escalate substantially.
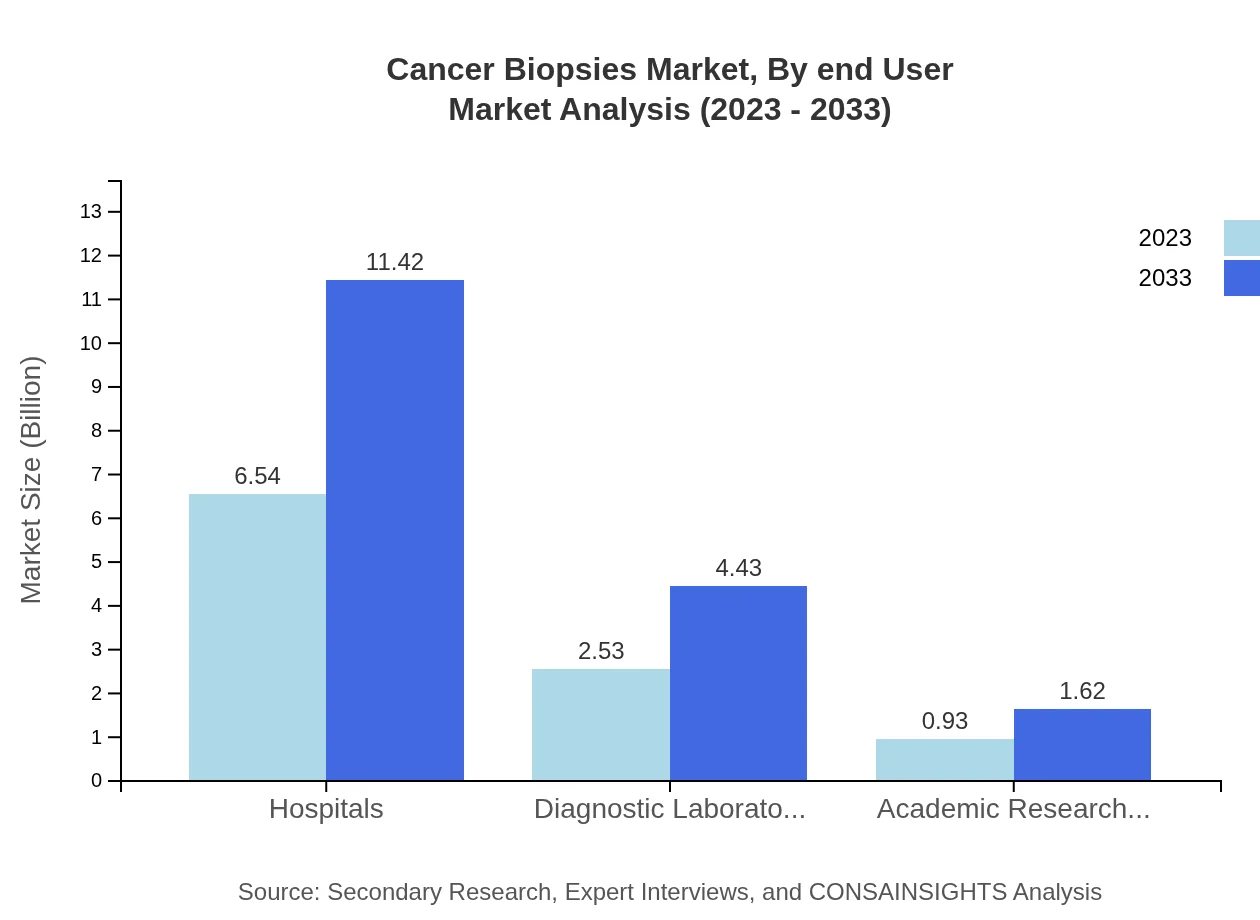
Cancer Biopsies Market Analysis By End User
Hospitals remain the leading end-user, with a market size of $6.54 billion in 2023 and expected growth to $11.42 billion by 2033 due to high patient footfall and advanced diagnostics. Diagnostic Laboratories and Academic Research Institutions contribute significantly, with market sizes of $2.53 billion and $0.93 billion respectively.
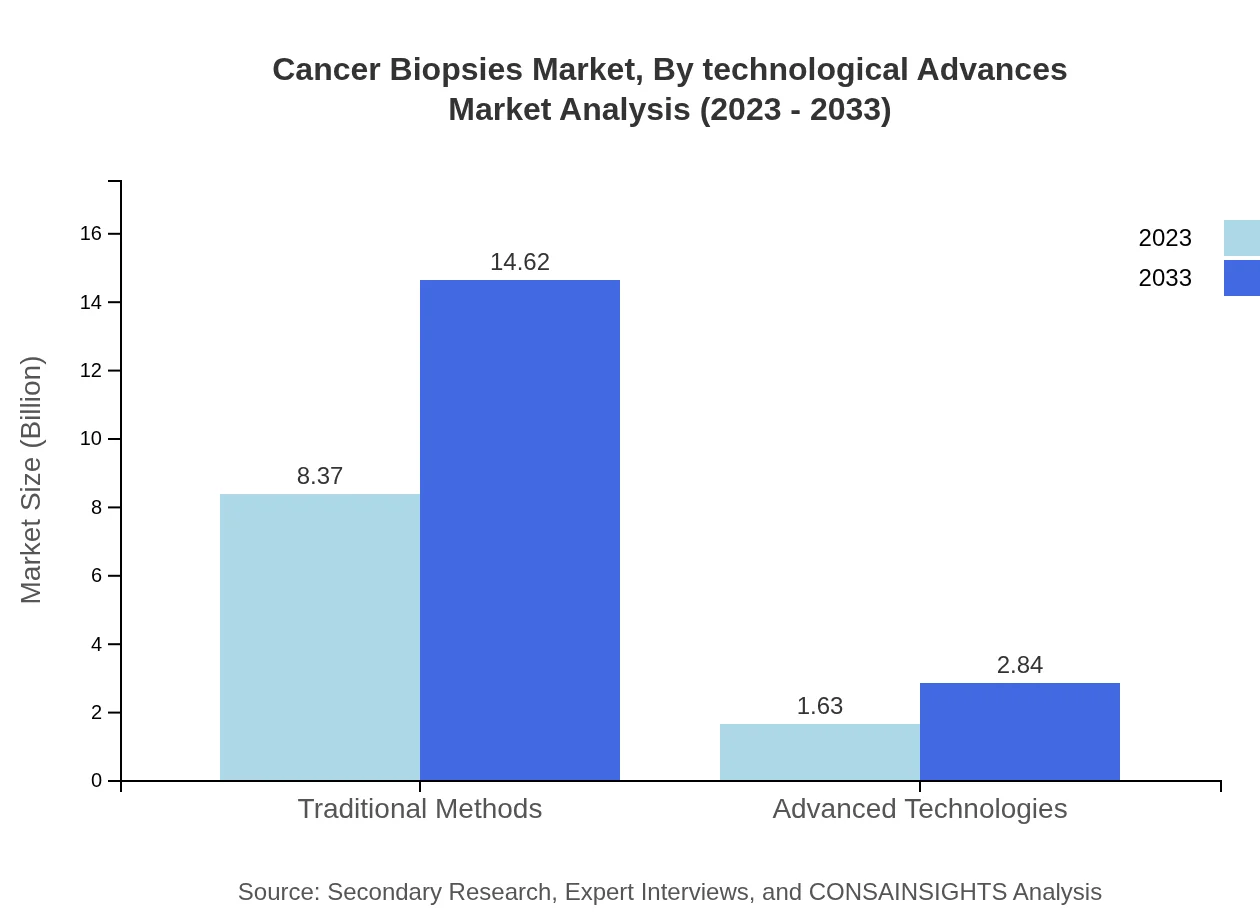
Cancer Biopsies Market Analysis By Technological Advances
Traditional Methods capture the majority share of the market at 83.73% in 2023, amounting to $8.37 billion, while Advanced Technologies represent a growing segment, projected to reach $2.84 billion driven by innovation and shifting industry standards.
Cancer Biopsies Market Analysis By Region
Market dynamics vary significantly by region, with North America, Europe, and Asia Pacific leading in demand due to advancements in healthcare systems, regulations, and infrastructure for conducting biopsies efficiently.
Cancer Biopsies Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cancer Biopsies Industry
Roche Diagnostics:
A leader in diagnostics, Roche specializes in innovative cancer diagnostic solutions, significantly contributing to the biopsy segment through advanced technologies.Thermo Fisher Scientific:
Well-known for its scientific instruments and medical diagnostic tests, Thermo Fisher plays a crucial role in biopsy technologies worldwide.Becton, Dickinson and Company (BD):
BD is a prominent player focusing on developing medical devices and diagnostics, significantly impacting the Cancer Biopsies market with its innovative products.Illumina, Inc.:
Illumina is at the forefront of genomic solutions, providing advanced liquid biopsy technologies to enhance cancer diagnosis and monitoring.Siemens Healthineers:
Siemens is renowned for its diagnostic imaging and laboratory diagnostics, contributing extensively to the evolution of biopsy procedures.We're grateful to work with incredible clients.









FAQs
What is the market size of cancer Biopsies?
The global cancer biopsies market is projected to grow from $10 billion in 2023 to a significant value by 2033, with a compound annual growth rate (CAGR) of 5.6%. This growth is driven by increasing cancer prevalence and the demand for accurate diagnostics.
What are the key market players or companies in the cancer Biopsies industry?
Key players in the cancer-biopsies market include leading healthcare firms specializing in diagnostics, research, and treatment technologies. Their innovative approaches and investment in advanced technologies significantly contribute to the market's growth and product offerings.
What are the primary factors driving the growth in the cancer Biopsies industry?
Growth drivers in the cancer biopsies market encompass rising cancer incidences, advancements in diagnostic technologies, increasing awareness about early detection, and the growing demand for precision medicine tailored to individual patient needs, enhancing treatment outcomes.
Which region is the fastest Growing in cancer Biopsies?
North America is the fastest-growing region in the cancer biopsies market, expected to expand significantly from $3.24 billion in 2023 to $5.65 billion by 2033. This growth is driven by technological innovations and high demand for oncology advancements.
Does ConsaInsights provide customized market report data for the cancer Biopsies industry?
Yes, ConsaInsights offers customized market report data for the cancer-biopsies industry. Clients benefit from tailored insights that address specific interests, regional dynamics, and unique market segments to enhance strategic decision-making.
What deliverables can I expect from this cancer Biopsies market research project?
Deliverables from the cancer-biopsies market research project include comprehensive reports on market trends, segmentation analysis, competitor insights, regional market dynamics, and future projections, enabling businesses to make informed strategic choices.
What are the market trends of cancer Biopsies?
Current trends in the cancer biopsies market include the shift towards liquid biopsies, integration of advanced technologies for improved accuracy, and a growing emphasis on personalized treatment approaches, reflecting the evolving landscape of cancer diagnostics.