Cancer Gene Therapy Market Report
Published Date: 31 January 2026 | Report Code: cancer-gene-therapy
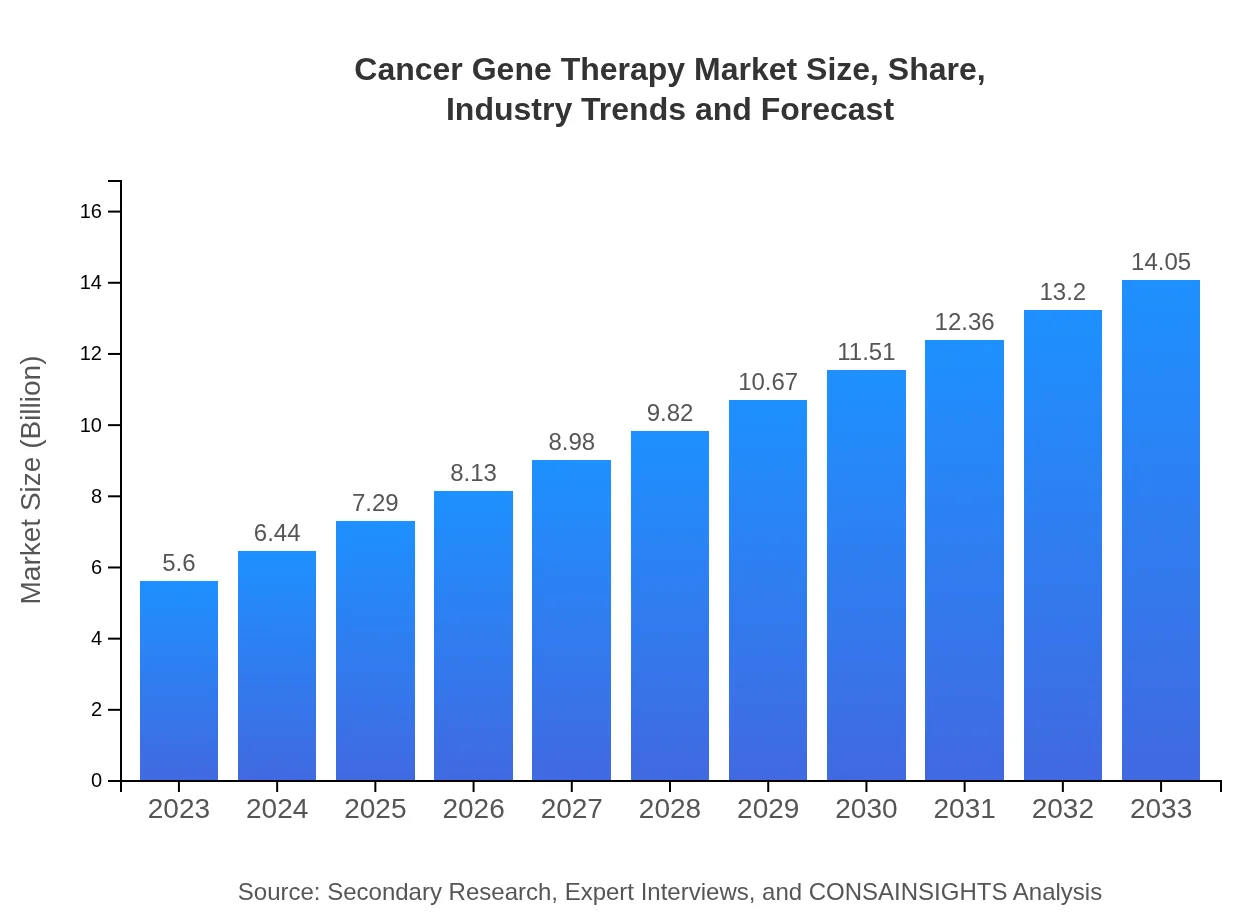
Cancer Gene Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Cancer Gene Therapy market, covering key insights, market forecasts from 2023 to 2033, and comprehensive assessments of various regions and segments within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.3% |
| 2033 Market Size | $14.05 Billion |
| Top Companies | Novartis AG, Gilead Sciences, Inc., Spark Therapeutics, Amgen, Inc. |
| Last Modified Date | 31 January 2026 |
Cancer Gene Therapy Market Overview
Customize Cancer Gene Therapy Market Report market research report
- ✔ Get in-depth analysis of Cancer Gene Therapy market size, growth, and forecasts.
- ✔ Understand Cancer Gene Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cancer Gene Therapy
What is the Market Size & CAGR of Cancer Gene Therapy market in 2033?
Cancer Gene Therapy Industry Analysis
Cancer Gene Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cancer Gene Therapy Market Analysis Report by Region
Europe Cancer Gene Therapy Market Report:
Europe's Cancer Gene Therapy market is estimated to expand from USD 1.61 billion in 2023 to USD 4.03 billion by 2033. Factors contributing to this growth include increasing rates of cancer diagnosis and ongoing clinical trials.Asia Pacific Cancer Gene Therapy Market Report:
In the Asia-Pacific region, the Cancer Gene Therapy market is projected to grow from USD 1.21 billion in 2023 to USD 3.03 billion by 2033, highlighting significant opportunities for market expansion driven by increasing healthcare investments and rising prevalence of cancer.North America Cancer Gene Therapy Market Report:
North America remains a dominant player in the Cancer Gene Therapy market, with a forecast size of USD 4.84 billion by 2033, growing from USD 1.93 billion in 2023. The region's growth is supported by strong R&D activities, high healthcare expenditure, and the presence of key industry players.South America Cancer Gene Therapy Market Report:
South America is expected to see modest growth in the Cancer Gene Therapy market, rising from USD 0.31 billion in 2023 to USD 0.77 billion by 2033. The market growth is fostered by government initiatives aimed at improving healthcare infrastructure and cancer treatment facilities.Middle East & Africa Cancer Gene Therapy Market Report:
The Middle East and Africa market is expected to steadily grow from USD 0.55 billion in 2023 to USD 1.38 billion by 2033. Growth will be driven by the expansion of healthcare services and investments in genomic research.Tell us your focus area and get a customized research report.
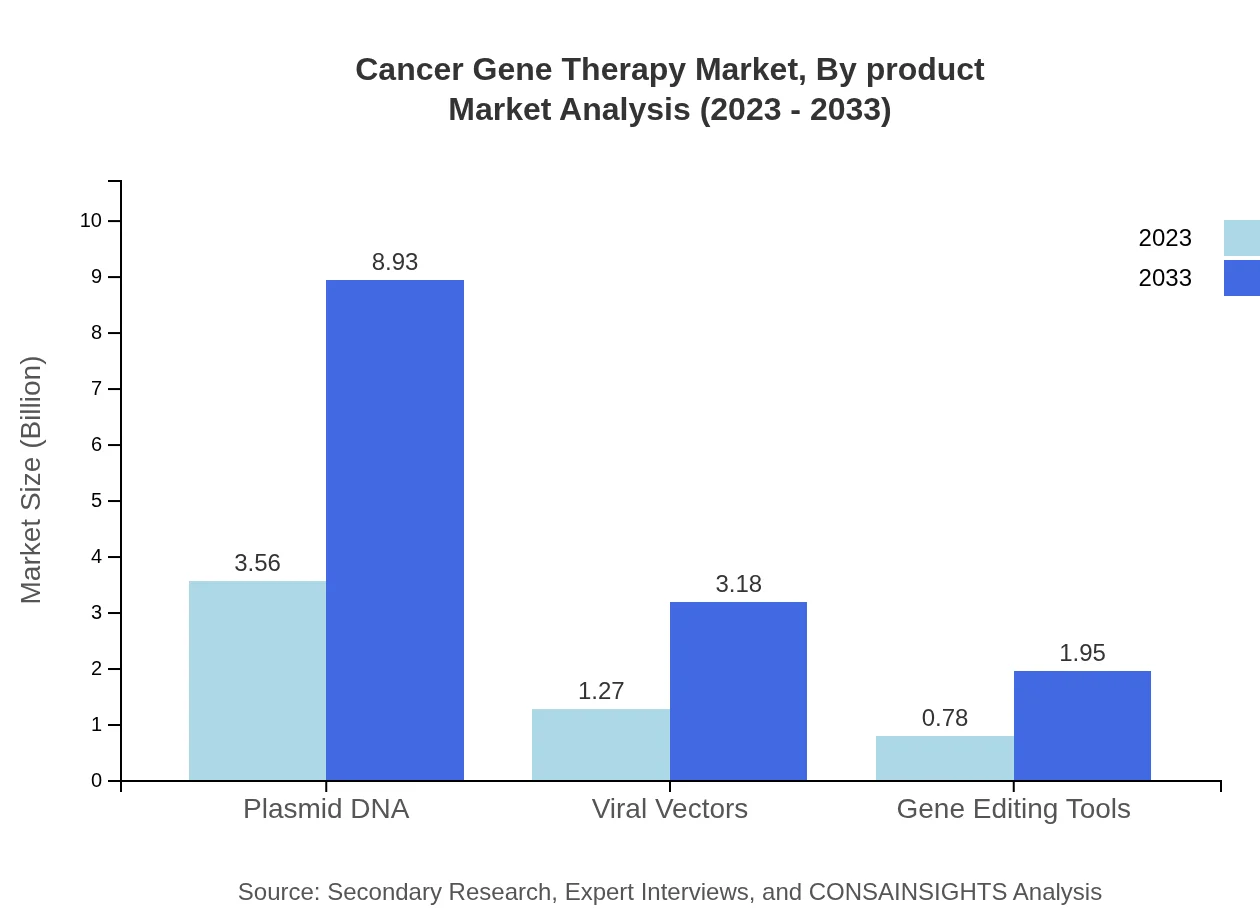
Cancer Gene Therapy Market Analysis By Product
The Cancer Gene Therapy market by product type encompasses various segments, with Plasmid DNA dominating the market size, projected to reach USD 8.93 billion by 2033 from USD 3.56 billion in 2023, capturing a substantial market share of 63.54%. Viral Vectors and Gene Editing Tools are also prominent, with estimated sizes of USD 3.18 billion and USD 1.95 billion, respectively, by 2033.
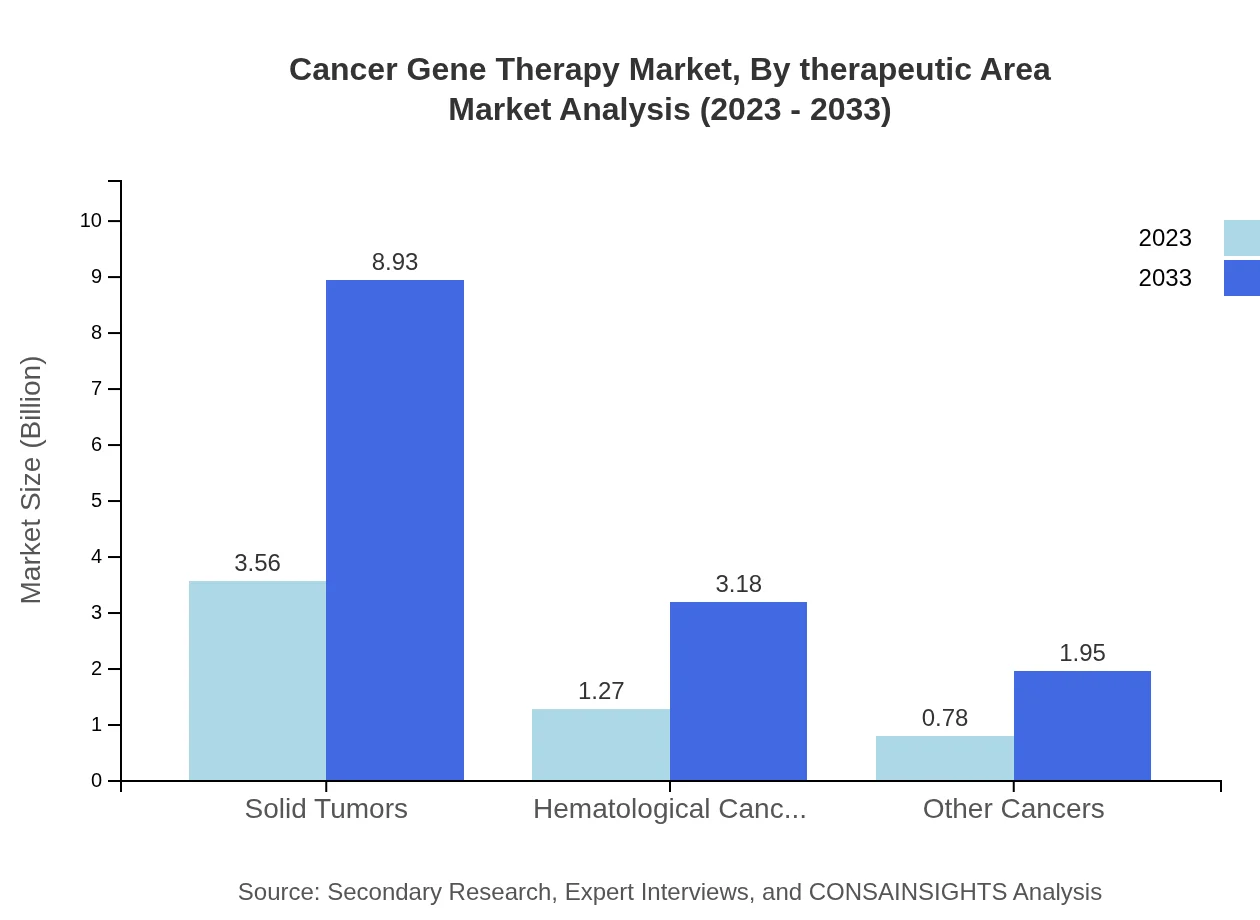
Cancer Gene Therapy Market Analysis By Therapeutic Area
By therapeutic area, Solid Tumors will retain the largest market allocation, increasing from USD 3.56 billion in 2023 to USD 8.93 billion by 2033, representing 63.54% market share. Hematological Cancers and Other Cancers will also contribute significantly to market growth.
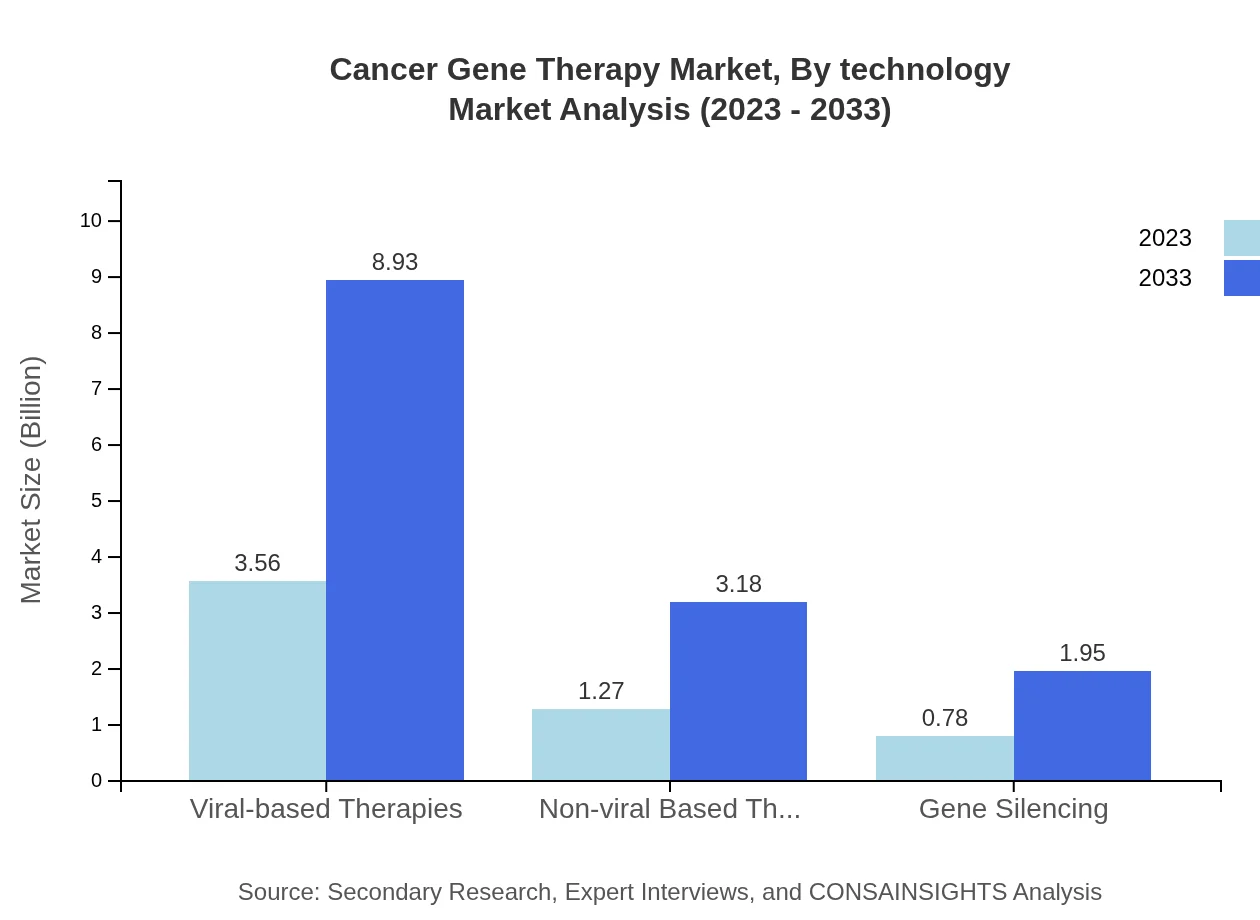
Cancer Gene Therapy Market Analysis By Technology
The market is split between Viral-based and Non-viral Based Therapies, with Viral-based Therapies expected to yield USD 8.93 billion by 2033 from USD 3.56 billion in 2023. Additionally, gene silencing techniques are expected to grow, capturing a noteworthy share in future developments.
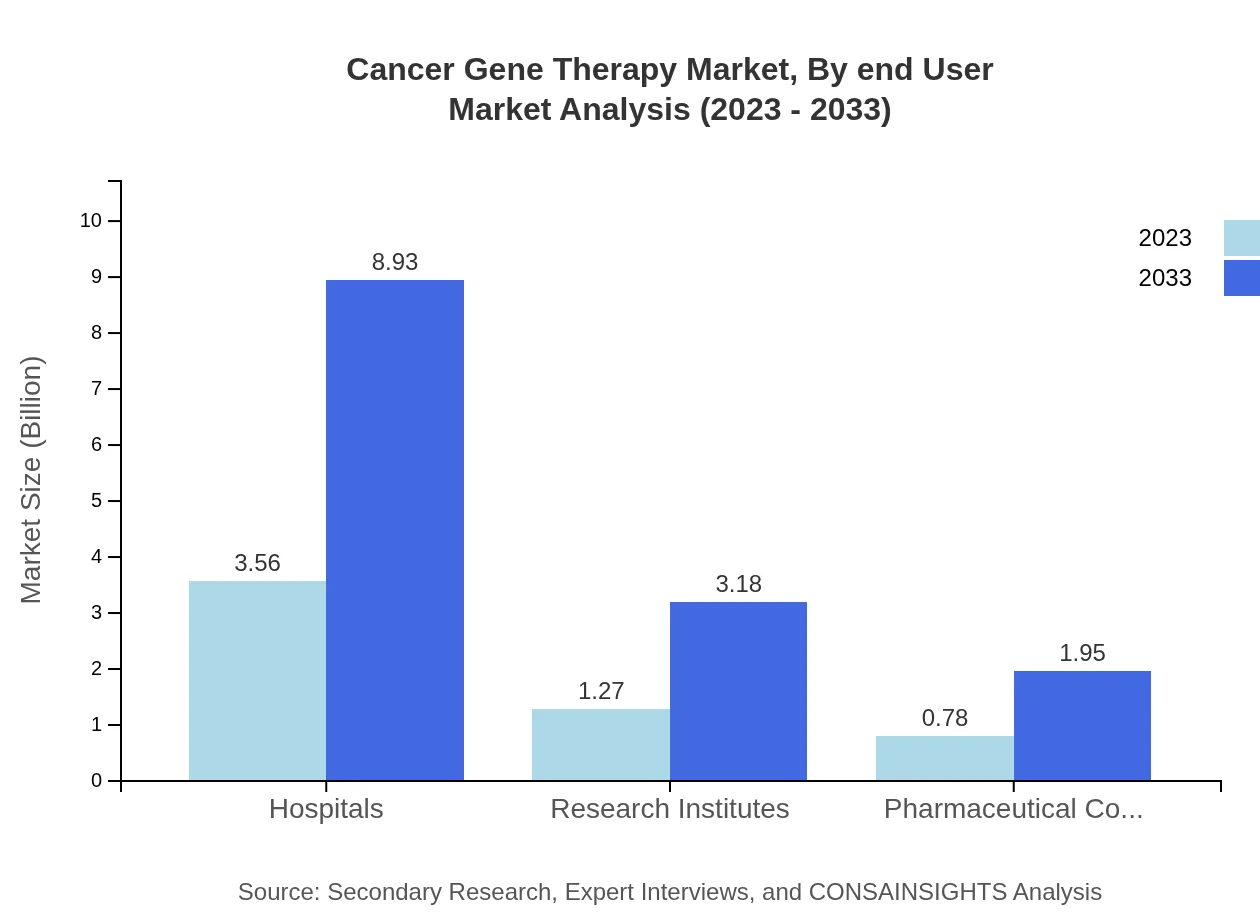
Cancer Gene Therapy Market Analysis By End User
Hospitals are projected to remain the leading end-user segment, with market size rising from USD 3.56 billion in 2023 to USD 8.93 billion by 2033, demonstrating the industry's focus on delivering care and treatments directly to patients within clinical settings.
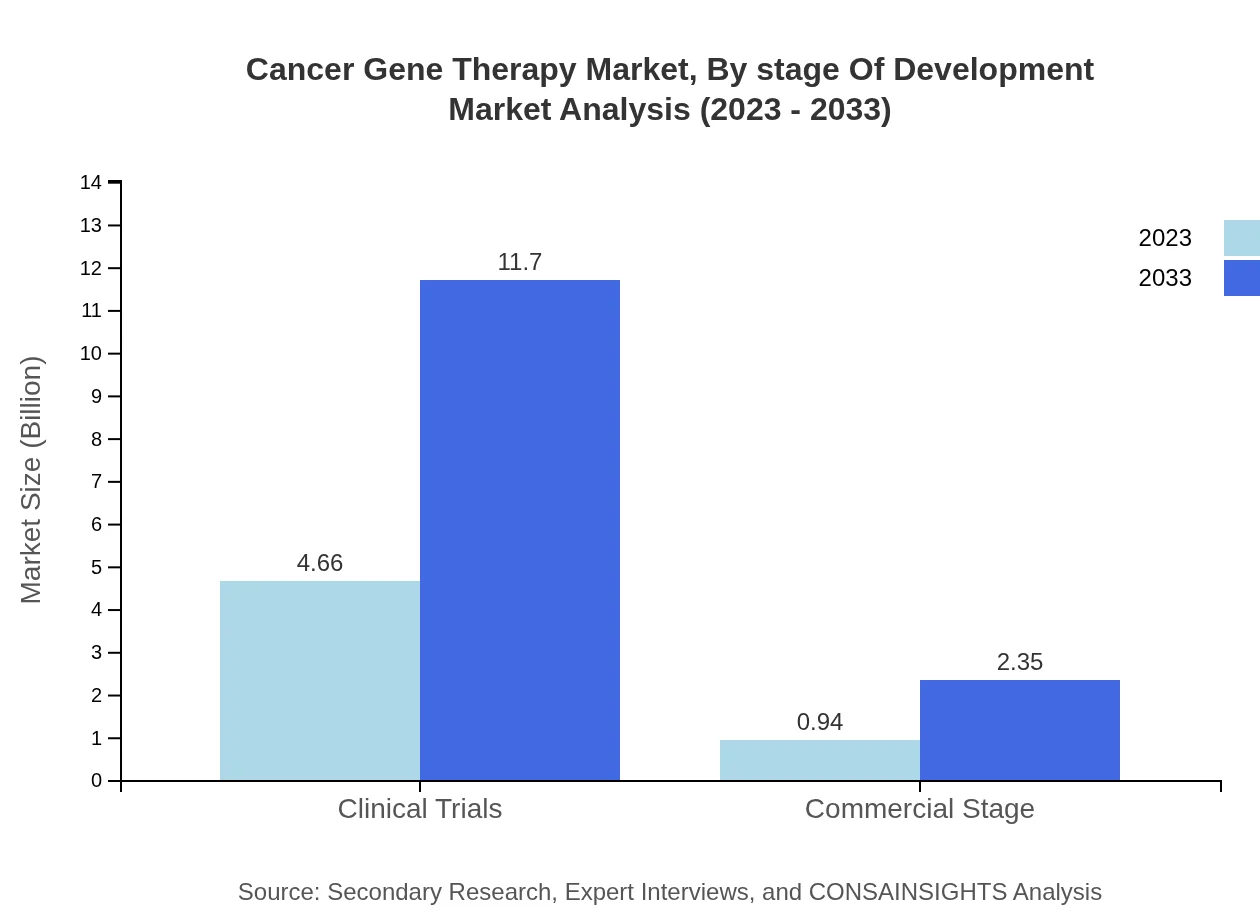
Cancer Gene Therapy Market Analysis By Stage Of Development
In terms of development stage, Clinical Trials currently account for a substantial market size of USD 4.66 billion, expected to grow to USD 11.70 billion by 2033. This indicates the emphasis on advancing clinical research to transition therapies from lab to commercial markets.
Cancer Gene Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cancer Gene Therapy Industry
Novartis AG:
Novartis is a leading global healthcare company focused on innovative medicines and has made significant advances in CAR-T cell therapy for hematological cancers.Gilead Sciences, Inc.:
Gilead specializes in antiviral drugs but has entered the gene therapy space, particularly with CAR-T therapeutics for treating various cancers.Spark Therapeutics:
This biotechnology firm focuses on developing therapies for genetic diseases, with gene therapy products aimed at rare genetic disorders and significant cancer treatments.Amgen, Inc.:
Amgen is a pioneer in biopharmaceutical manufacturing, continually investing in gene therapies, particularly for oncology.We're grateful to work with incredible clients.









FAQs
What is the market size of cancer Gene Therapy?
The global cancer gene therapy market was valued at approximately $5.6 billion in 2023, with a projected CAGR of 9.3%, indicating robust growth prospects as advancements in gene therapy technology continue to evolve over the next decade.
What are the key market players or companies in this cancer Gene Therapy industry?
Key players in the cancer gene therapy market include major pharmaceutical and biotech companies engaged in innovative therapies, development of viral vectors, and collaboration with research institutes to expand their portfolio and market presence.
What are the primary factors driving the growth in the cancer Gene Therapy industry?
Growth in the cancer gene therapy industry is driven by increasing cancer prevalence, advancements in genetic research, technological innovations in gene editing tools, and a growing focus on personalized medicine and targeted therapies.
Which region is the fastest Growing in the cancer Gene Therapy?
The Asia Pacific region is identified as the fastest-growing market for cancer gene therapy, with market size projected to increase from $1.21 billion in 2023 to $3.03 billion by 2033, reflecting a significant growth trajectory driven by expanding healthcare infrastructure.
Does ConsaInsights provide customized market report data for the cancer Gene Therapy industry?
Yes, ConsaInsights offers customized market reports tailored to specific needs and requirements, providing in-depth analysis and insights into the cancer gene therapy industry, accommodating diverse stakeholder interests.
What deliverables can I expect from this cancer Gene Therapy market research project?
Deliverables from the cancer gene therapy market research project include comprehensive reports, regional market analysis, segmentation insights, and actionable recommendations to support strategic decision-making and investment planning.
What are the market trends of cancer Gene Therapy?
Current trends in the cancer gene therapy market include increasing investment in clinical trials, the emergence of viral-based therapies, and a focus on innovative delivery systems to enhance treatment efficacy and patient outcomes.