Capecitabine Market Report
Published Date: 31 January 2026 | Report Code: capecitabine
Capecitabine Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Capecitabine market, covering key metrics, forecast from 2023 to 2033, market trends, regional insights, and competitive landscape. It aims to equip stakeholders with critical insights for strategic planning and investment decisions.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
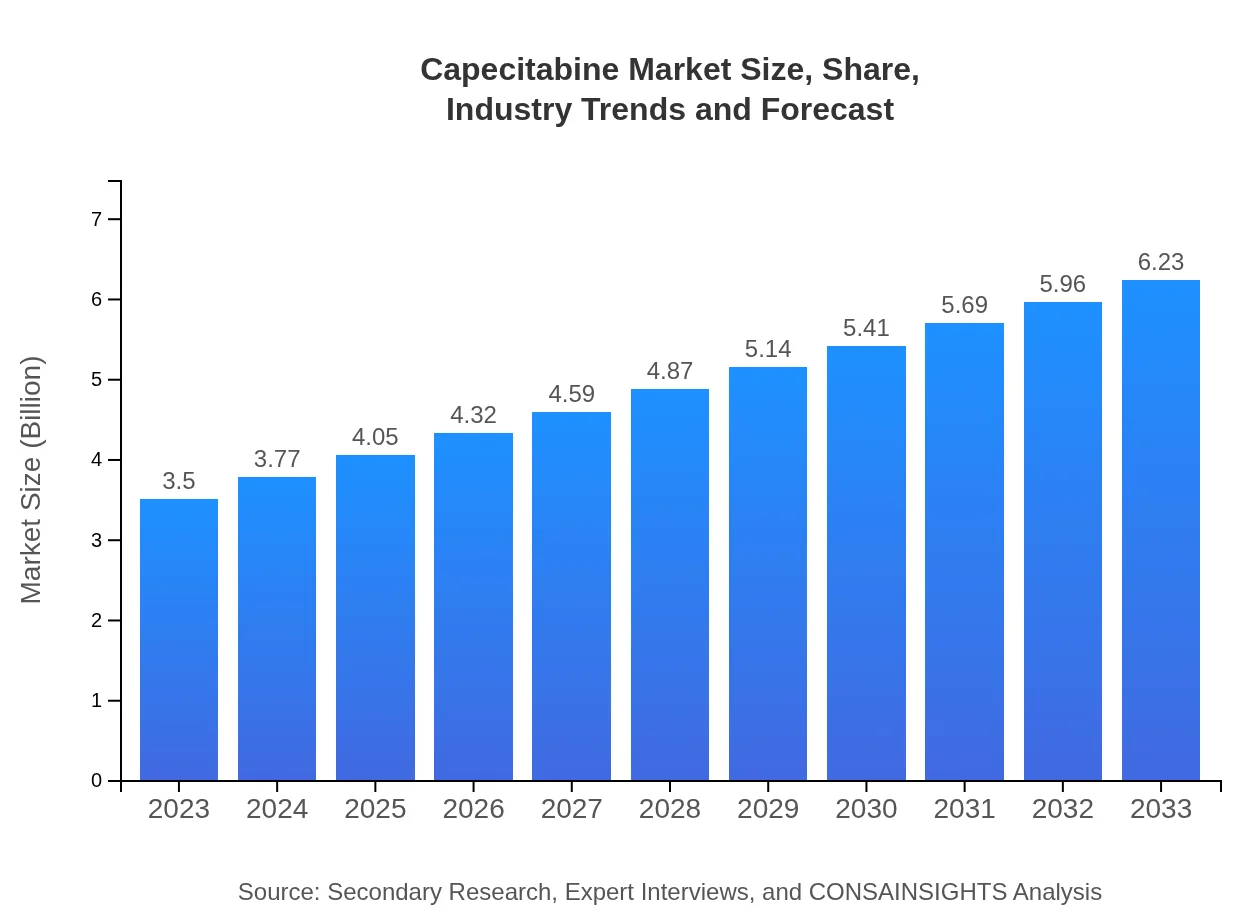
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 5.8% |
| 2033 Market Size | $6.23 Billion |
| Top Companies | Roche, Teva Pharmaceuticals, Pfizer , Novartis |
| Last Modified Date | 31 January 2026 |
Capecitabine Market Overview
Customize Capecitabine Market Report market research report
- ✔ Get in-depth analysis of Capecitabine market size, growth, and forecasts.
- ✔ Understand Capecitabine's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Capecitabine
What is the Market Size & CAGR of Capecitabine market in 2023?
Capecitabine Industry Analysis
Capecitabine Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Capecitabine Market Analysis Report by Region
Europe Capecitabine Market Report:
The European market for Capecitabine is expected to rise from $0.87 billion in 2023 to $1.54 billion by 2033, supported by favorable reimbursement policies and awareness programs focused on cancer therapies.Asia Pacific Capecitabine Market Report:
The Asia Pacific Capecitabine market is valued at $0.75 billion in 2023 and is projected to reach $1.33 billion by 2033, driven by increasing disease awareness and improving healthcare infrastructure.North America Capecitabine Market Report:
North America leads the market with an expected growth from $1.36 billion in 2023 to $2.42 billion in 2033. The driver includes advanced healthcare facilities, a high prevalence of breast and colorectal cancer, and strong support for research.South America Capecitabine Market Report:
In South America, the Capecitabine market is expected to grow from $0.30 billion in 2023 to $0.54 billion by 2033. Increased availability of oncology services and affordability of generic drugs contribute to this growth.Middle East & Africa Capecitabine Market Report:
The Middle East and Africa region sees an increase from $0.23 billion in 2023 to $0.41 billion by 2033, attributed to collaborations between governments and international organizations to improve cancer care.Tell us your focus area and get a customized research report.
Capecitabine Market Analysis By Application
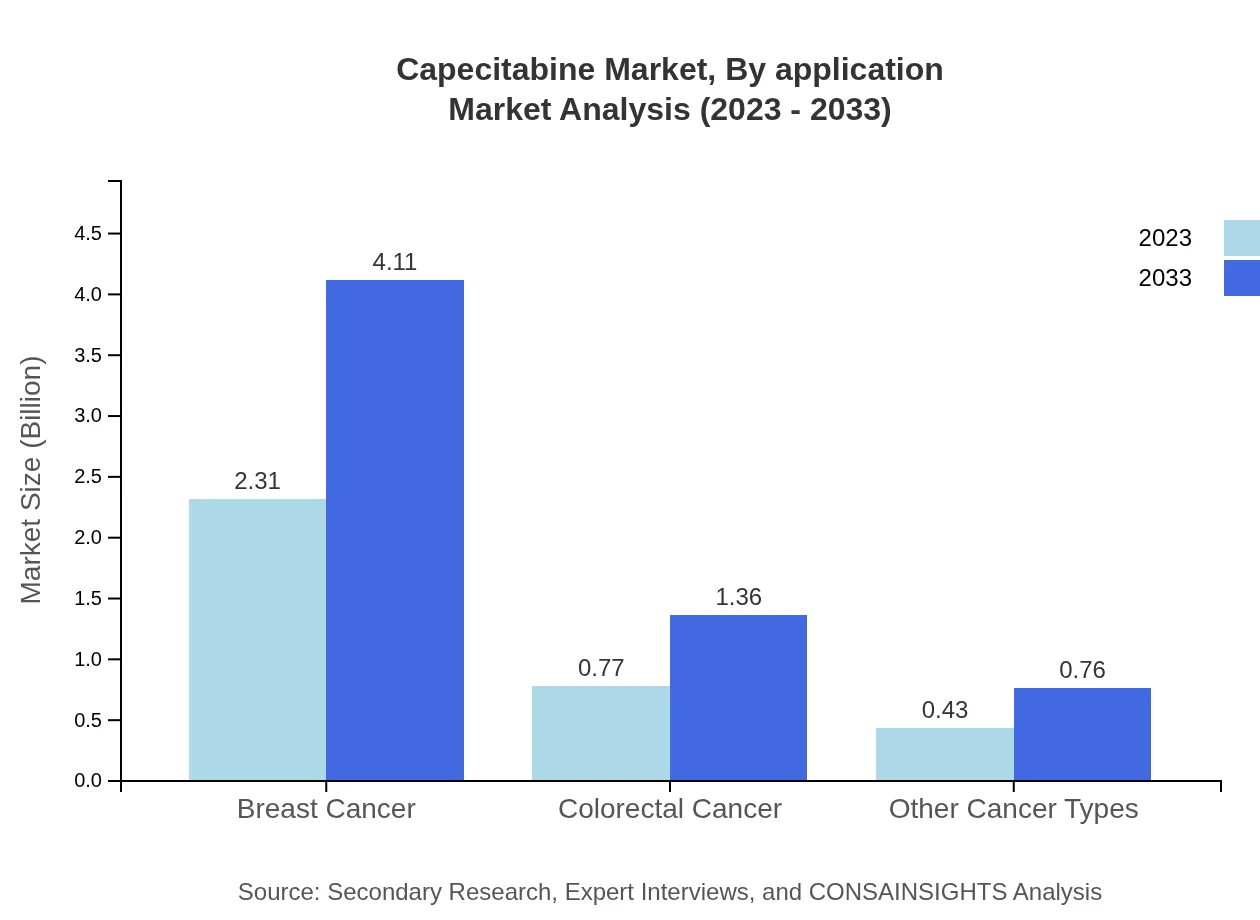
The application-based segmentation shows a significant share for Breast Cancer, which accounted for a market size of $2.31 billion in 2023 and is projected to reach $4.11 billion by 2033. Colorectal Cancer follows with a size of $0.77 billion in 2023, forecasted to grow to $1.36 billion. Other Cancer Types include diverse malignancies that will generate $0.43 billion, projected at $0.76 billion by 2033.
Capecitabine Market Analysis By Drug Formulation
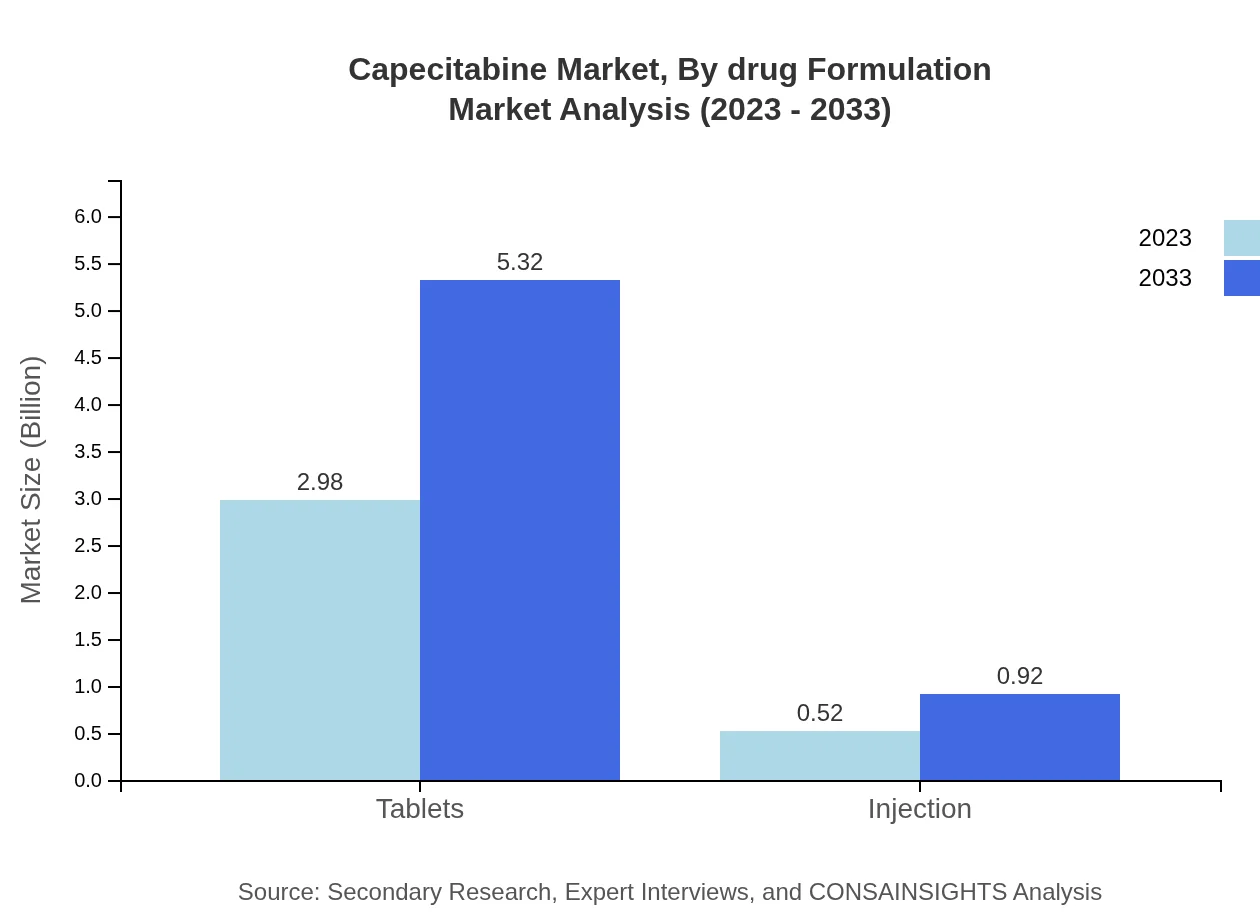
In terms of drug formulation, Tablets remain the predominant form, encompassing a market size of $2.98 billion in 2023, expected to rise to $5.32 billion by 2033. Injections account for a smaller fraction of the market, starting at $0.52 billion in 2023 and likely reaching $0.92 billion by 2033. The preference for tablets reflects greater patient convenience and adherence.
Capecitabine Market Analysis By Distribution Channel
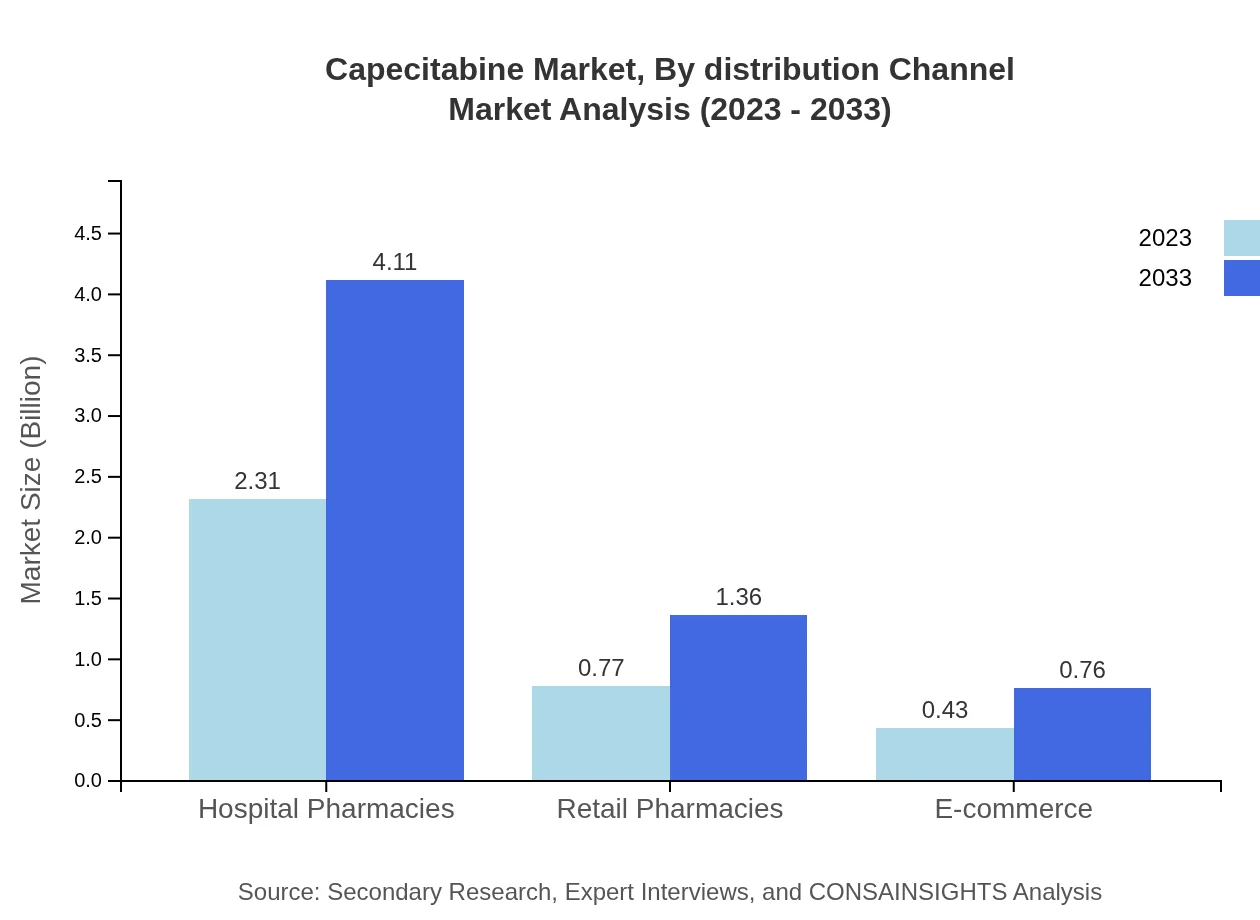
Distribution channels reveal that Hospital Pharmacies dominate, holding a market size of $2.31 billion in 2023, growing to $4.11 billion by 2033. Retail Pharmacies and E-commerce are important channels, with retail accounting for $0.77 billion, anticipated to reach $1.36 billion, while e-commerce is projected at $0.43 billion, growing to $0.76 billion respectively.
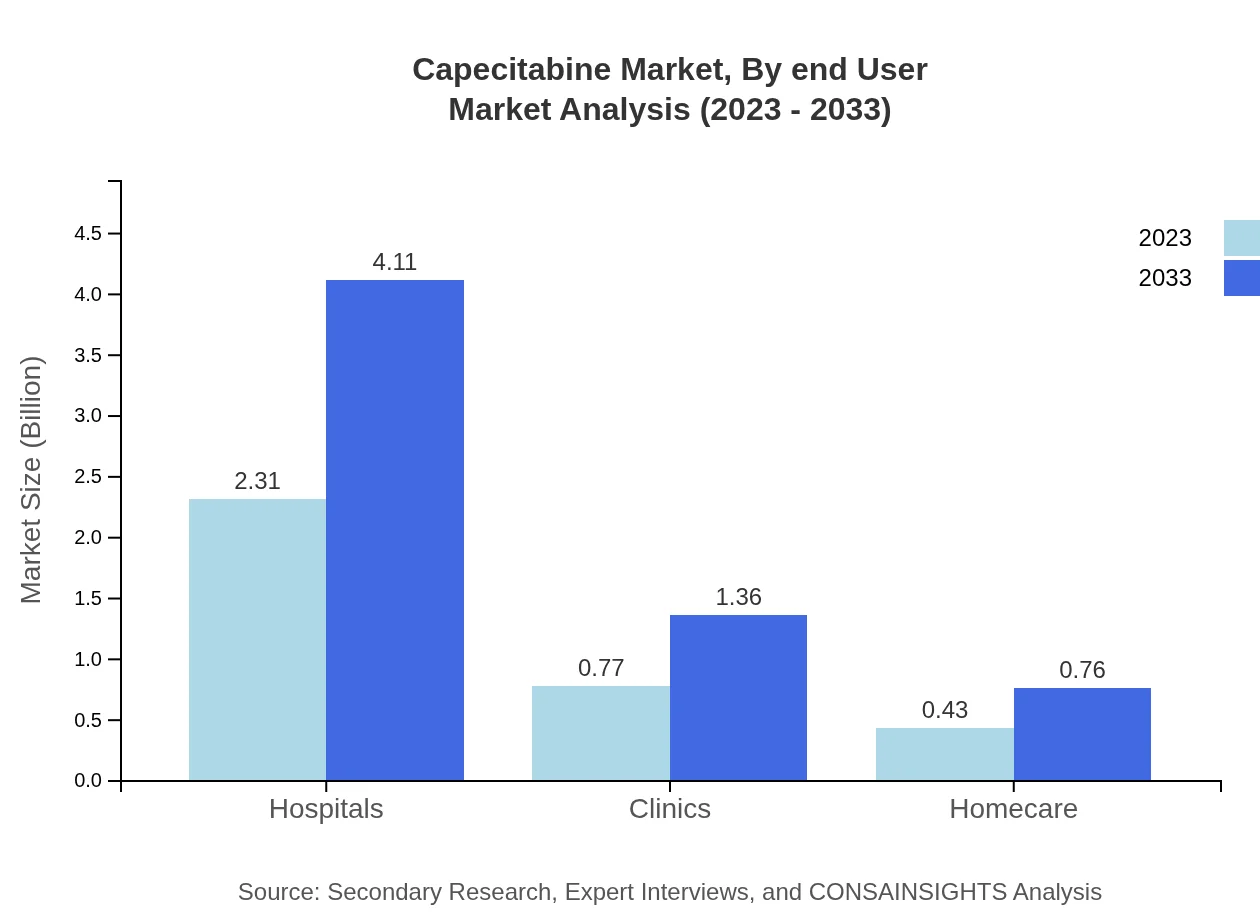
Capecitabine Market Analysis By End User
Hospitals constitute the leading end-user segment due to their integrated treatment capabilities, projected to hold 65.89% share consistently throughout the forecast period. Clinics and homecare settings show increasing trends, holding a combined share of 34.11%, reflecting evolving cancer care paradigms focusing on outpatient services.
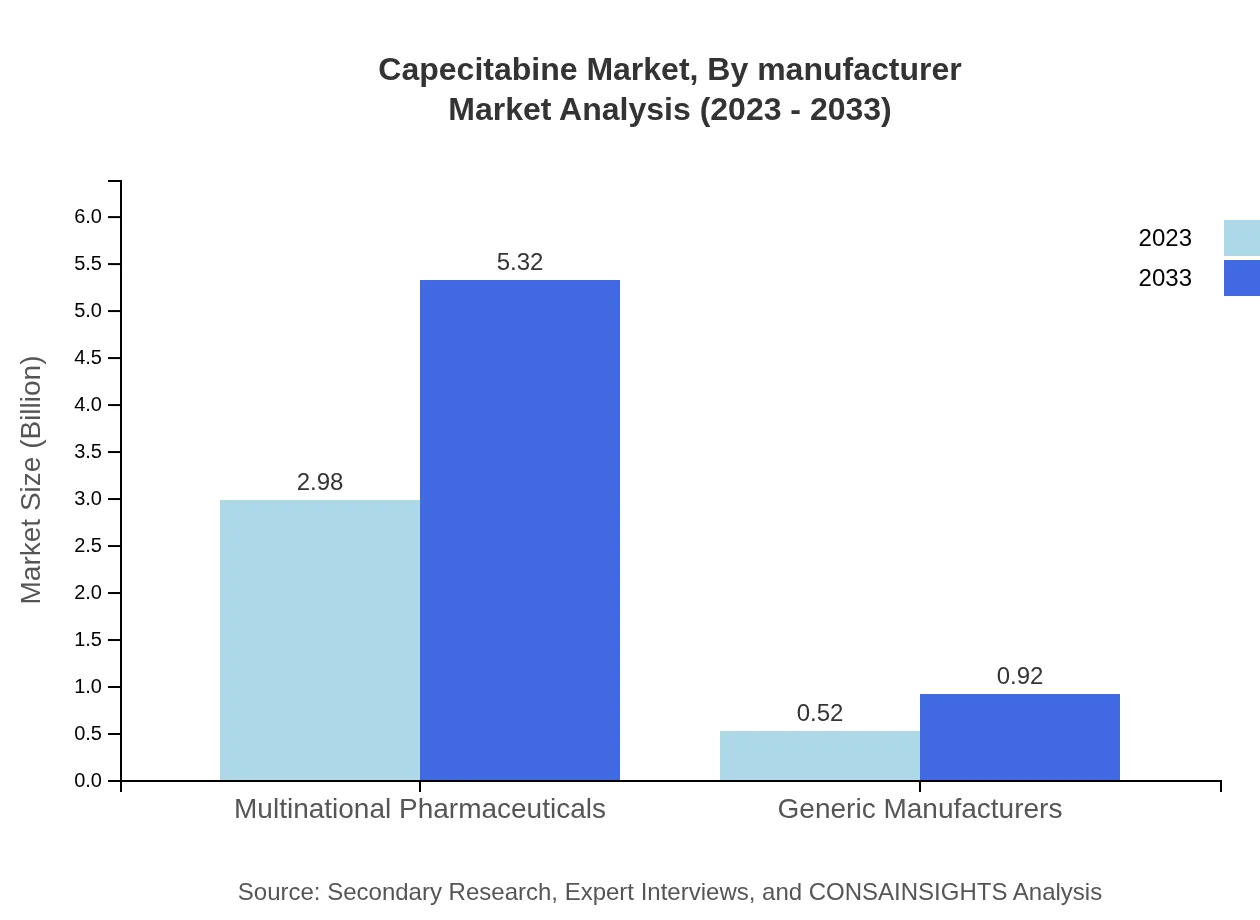
Capecitabine Market Analysis By Manufacturer
Multinational pharmaceutical companies lead the market, making up 85.26% of the market share in 2023 and expected to maintain this dominance until 2033. Generic manufacturers, who hold a 14.74% share, play a crucial role in enhancing access and affordability to therapeutic options in emerging economies.
Capecitabine Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Capecitabine Industry
Roche:
A global leader in pharmaceuticals, Roche is renowned for its innovative approaches in oncology, including the development of Capecitabine, enhancing cancer care and treatment protocols.Teva Pharmaceuticals:
Teva is a major player in the generic drug market with significant contributions to the distribution of Capecitabine, providing affordable access to this critical cancer treatment.Pfizer :
Pfizer stands out for its extensive research in oncology, with Capecitabine among its portfolio for effective cancer management, supported by robust clinical research.Novartis:
Novartis is actively involved in developing oncology products, including Capecitabine, focusing on improving patient outcomes through innovative formulations and new therapy protocols.We're grateful to work with incredible clients.









FAQs
What is the market size of capecitabine?
The capecitabine market is projected to reach a size of $3.5 billion by 2033, with a CAGR of 5.8% from 2023. This growth is driven by increasing cancer prevalence and advancements in treatment methodologies.
What are the key market players or companies in the capecitabine industry?
Key players in the capecitabine market include multinational pharmaceutical companies that dominate the landscape, particularly focusing on oncology treatments. They contribute significantly to innovation and market share within the growing segment.
What are the primary factors driving the growth in the capecitabine industry?
Growth in the capecitabine segment is fueled by rising incidence rates of breast and colorectal cancers, continuous advancements in drug formulations, and increasing adoption of oral chemotherapy medications among patients.
Which region is the fastest Growing in the capecitabine market?
The Asia Pacific region is the fastest-growing market for capecitabine, expected to grow from $0.75 billion in 2023 to $1.33 billion by 2033, driven by improving healthcare infrastructure and rising cancer incidence.
Does ConsaInsights provide customized market report data for the capecitabine industry?
Yes, ConsaInsights offers tailored market report data services for the capecitabine industry, ensuring clients receive insights that meet their specific requirements, enabling effective decision-making.
What deliverables can I expect from this capecitabine market research project?
Clients can expect comprehensive reports, including market analysis, segmentation data, regional insights, trends, and forecasts, along with actionable strategies that can drive growth in the capecitabine market.
What are the market trends of capecitabine?
Current trends in the capecitabine market include a shift towards increased oral chemotherapy options, heightened focus on personalized medicine, and a growing emphasis on affordability and accessibility of cancer treatments.