Car T Cell Therapy Market Report
Published Date: 31 January 2026 | Report Code: car-t-cell-therapy
Car T Cell Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Car T Cell Therapy market, including insights into market dynamics, regional developments, segment performance, and future trends projected from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
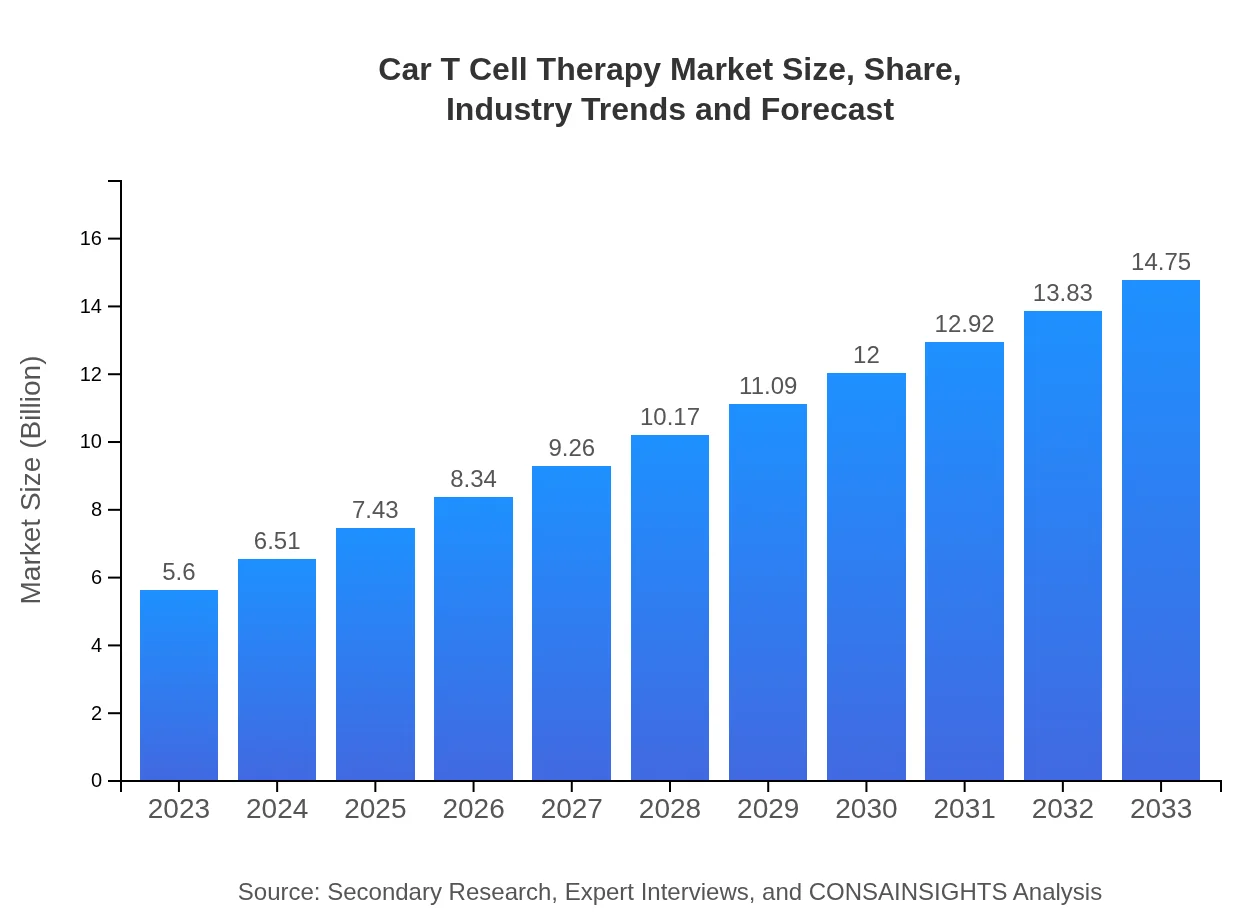
| 2023 Market Size | $5.60 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $14.75 Billion |
| Top Companies | Novartis, Gilead Sciences, Bristol-Myers Squibb, Amgen |
| Last Modified Date | 31 January 2026 |
Car T Cell Therapy Market Overview
Customize Car T Cell Therapy Market Report market research report
- ✔ Get in-depth analysis of Car T Cell Therapy market size, growth, and forecasts.
- ✔ Understand Car T Cell Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Car T Cell Therapy
What is the Market Size & CAGR of Car T Cell Therapy market in 2023?
Car T Cell Therapy Industry Analysis
Car T Cell Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Car T Cell Therapy Market Analysis Report by Region
Europe Car T Cell Therapy Market Report:
Europe's market is projected to expand from $1.38 billion in 2023 to approximately $3.63 billion by 2033. Ongoing clinical trials and government support for advanced cancer therapies are critical factors in this growth.Asia Pacific Car T Cell Therapy Market Report:
In 2023, the Car T Cell Therapy market in Asia Pacific is estimated at $1.23 billion, projected to grow to $3.23 billion by 2033. Increasing healthcare investments and growing acceptance of advanced therapies underscore this growth, alongside rising cancer incidences in the region.North America Car T Cell Therapy Market Report:
North America remains a leading market, valued at approximately $1.88 billion in 2023 and expected to grow to $4.95 billion by 2033. The presence of major market players, advanced infrastructure, and robust regulatory support contribute to its dominance.South America Car T Cell Therapy Market Report:
The market in South America is starting at $0.42 billion in 2023, anticipated to reach $1.11 billion by 2033. Although growth is slower compared to other regions, increased awareness and access to innovative treatments are beginning to drive demand.Middle East & Africa Car T Cell Therapy Market Report:
In this region, the market is estimated at $0.69 billion in 2023 and is expected to grow to $1.82 billion by 2033. Improved healthcare infrastructure and rising investments in medical technology are fostering this trajectory.Tell us your focus area and get a customized research report.
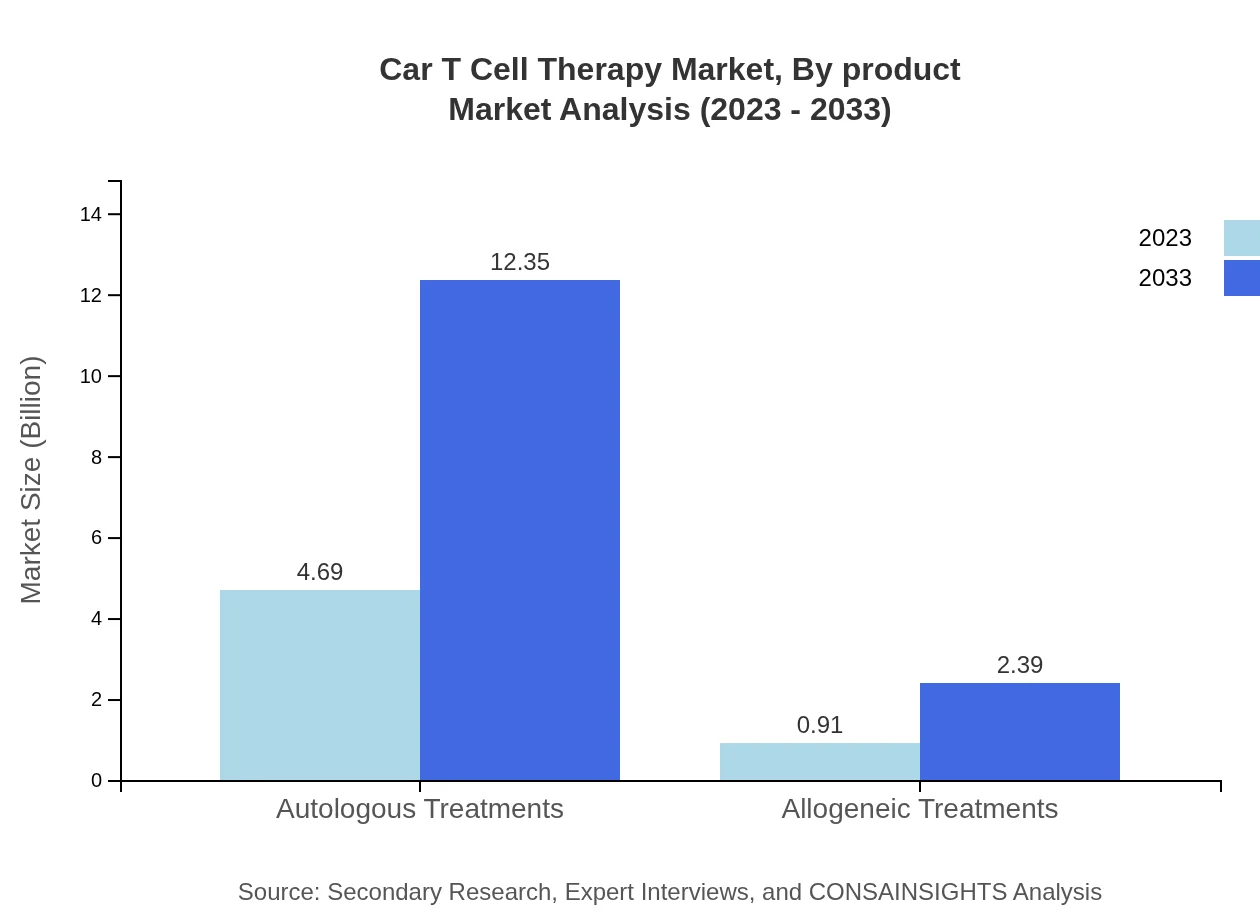
Car T Cell Therapy Market Analysis By Product
Autologous treatments currently hold a dominant market size of $4.69 billion in 2023 with an expected size of $12.35 billion by 2033, exhibiting a share of 83.78%. Allogeneic treatments, while smaller, show growth potential, starting at $0.91 billion in 2023 and reaching $2.39 billion by 2033 (16.22% share).
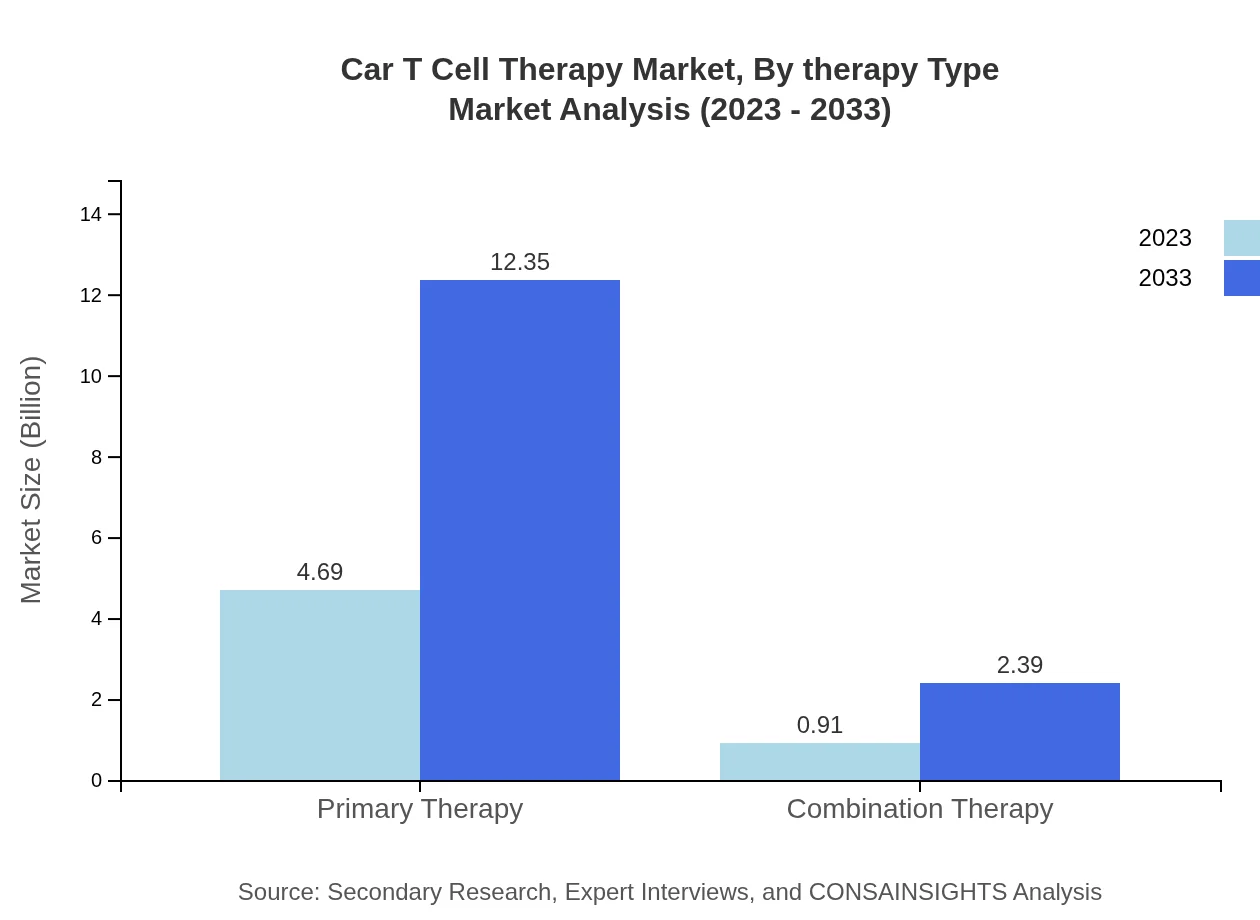
Car T Cell Therapy Market Analysis By Therapy Type
The primary therapy accounts for $4.69 billion in 2023 and is expected to grow to $12.35 billion by 2033, maintaining an 83.78% share. Combination therapies start at $0.91 billion in 2023 and are projected to reach $2.39 billion by 2033, holding a 16.22% market share.
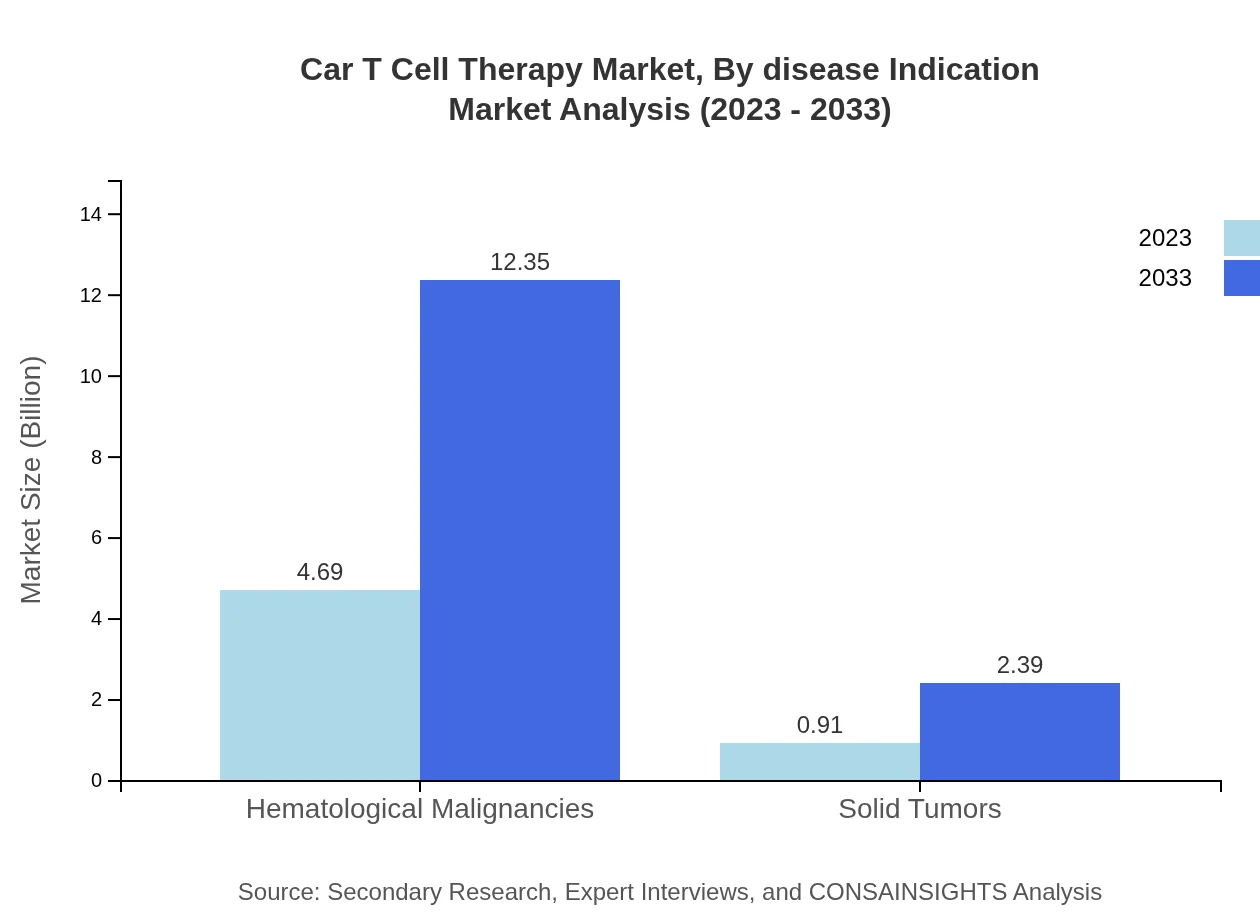
Car T Cell Therapy Market Analysis By Disease Indication
Hematological malignancies are predominant in the CAR-T market, valued at $4.69 billion in 2023, projected to reach $12.35 billion by 2033, capturing 83.78% of the market. Solid tumor therapies begin at $0.91 billion and are estimated to grow to $2.39 billion by 2033, with a 16.22% share.
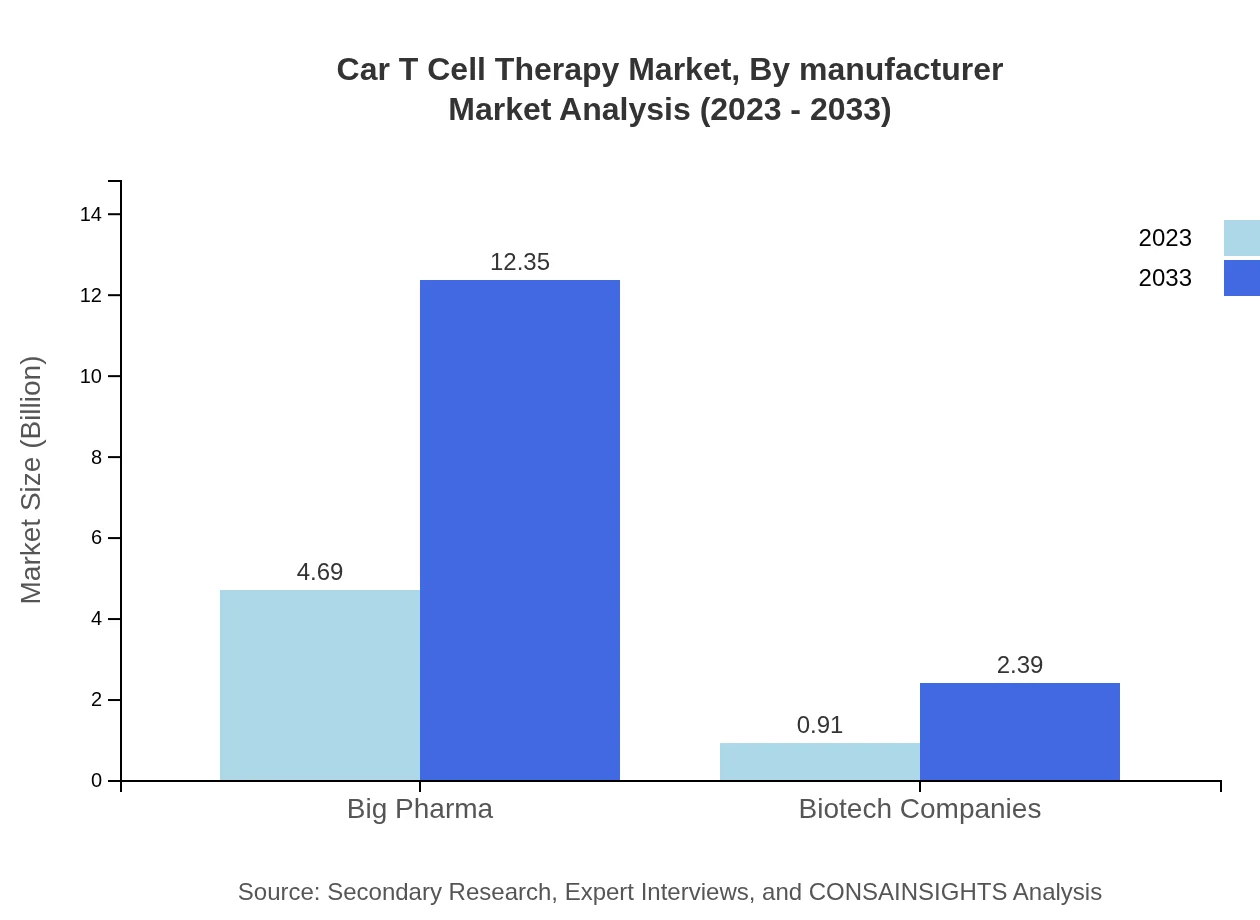
Car T Cell Therapy Market Analysis By Manufacturer
Big pharmaceutical companies constitute a market size of $4.69 billion in 2023, thriving with a projected growth to $12.35 billion by 2033, accounting for 83.78% of market share. Biotech companies, while smaller at $0.91 billion in 2023, are also expected to escalate to $2.39 billion by 2033, holding a 16.22% share.
Car T Cell Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Car T Cell Therapy Industry
Novartis:
Novartis is a leader in CAR-T therapy with its Kymriah product, focused on developing innovative therapies targeting hematologic malignancies.Gilead Sciences:
Gilead Sciences is noted for its Yescarta CAR-T product, a significant player in the market with ongoing research in advanced cell therapies.Bristol-Myers Squibb:
Bristol-Myers Squibb possesses a strong foothold in CAR-T therapy through its Breyanzi product, focusing on advancing treatments for various cancers.Amgen:
Amgen's innovative approaches in cell therapy, particularly targeted cancer treatments, contribute significantly to the CAR-T market landscape.We're grateful to work with incredible clients.









FAQs
What is the market size of car T Cell Therapy?
The global Car T Cell Therapy market was valued at $5.6 billion in 2023 and is projected to grow at a CAGR of 9.8%. This growth reflects advancements in therapeutic options and rising incidences of hematological malignancies.
What are the key market players or companies in this car T Cell Therapy industry?
Key players in the Car T Cell Therapy market include major pharmaceutical companies and biotechnology firms that focus on innovative therapies for cancer treatment. Their collaboration drives advancements in product development and market growth.
What are the primary factors driving the growth in the car T Cell Therapy industry?
Drivers of growth include the increasing prevalence of cancers, particularly hematological types, advancements in cell therapy techniques, and supportive regulatory frameworks that encourage development and approval of innovative therapies.
Which region is the fastest Growing in the car T Cell Therapy?
North America is the fastest-growing region for Car T Cell Therapy, with its market size expanding from $1.88 billion in 2023 to $4.95 billion by 2033. This growth is fueled by high healthcare expenditure and extensive research initiatives.
Does ConsaInsights provide customized market report data for the car T Cell Therapy industry?
Yes, ConsaInsights offers customized market report data for the Car T Cell Therapy industry. Clients can request tailored insights that cater to specific strategic planning and market assessment needs.
What deliverables can I expect from this car T Cell Therapy market research project?
Deliverables typically include comprehensive reports featuring market analysis, competitive landscape summaries, forecast data, regional insights, and segmentation analysis tailored to the client's needs.
What are the market trends of car T Cell Therapy?
Current trends in the Car T Cell Therapy market include a shift towards more personalized treatment approaches, collaborations between biotech and pharma companies, and the increasing adoption of novel therapies in treating rare cancers.