Cardiac Marker Testing Market Report
Published Date: 31 January 2026 | Report Code: cardiac-marker-testing
Cardiac Marker Testing Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the cardiac marker testing market, covering essential insights, trends, and projections from 2023 to 2033. It includes detailed examinations across various regions and segments, helping stakeholders understand market dynamics and future opportunities.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
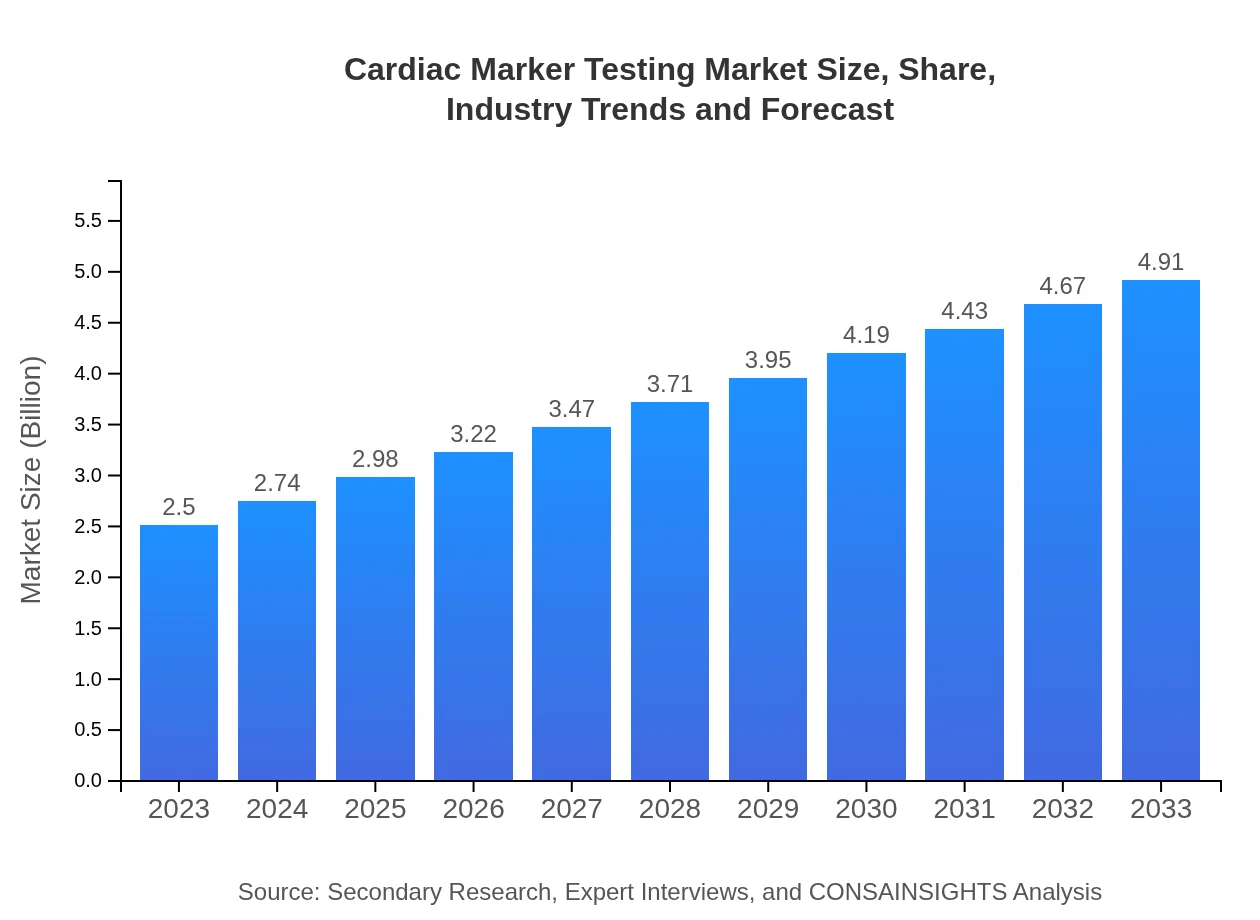
| 2023 Market Size | $2.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $4.91 Billion |
| Top Companies | Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Beckman Coulter, Thermo Fisher Scientific |
| Last Modified Date | 31 January 2026 |
Cardiac Marker Testing Market Overview
Customize Cardiac Marker Testing Market Report market research report
- ✔ Get in-depth analysis of Cardiac Marker Testing market size, growth, and forecasts.
- ✔ Understand Cardiac Marker Testing's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cardiac Marker Testing
What is the Market Size & CAGR of Cardiac Marker Testing market in 2023?
Cardiac Marker Testing Industry Analysis
Cardiac Marker Testing Market Segmentation and Scope
Tell us your focus area and get a customized research report.
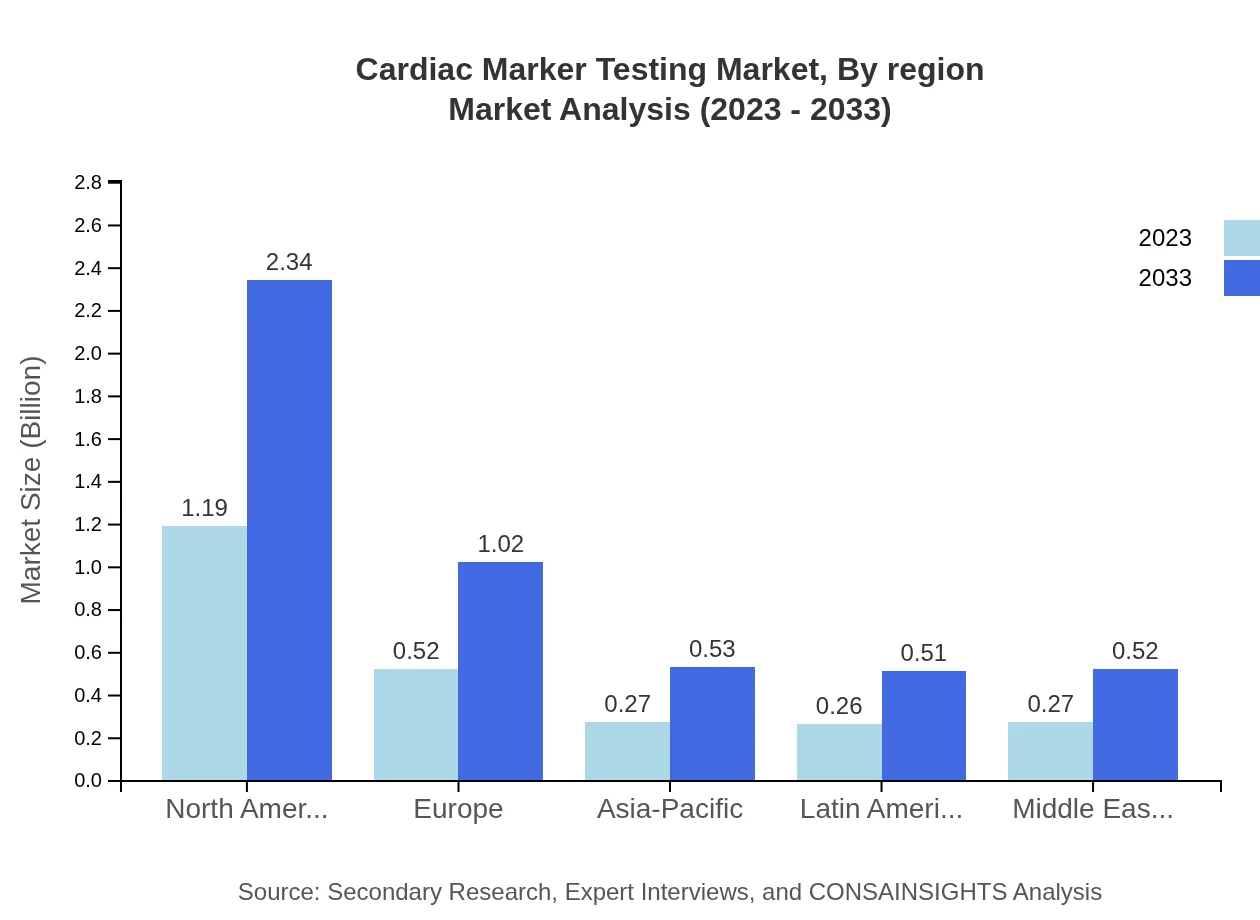
Cardiac Marker Testing Market Analysis Report by Region
Europe Cardiac Marker Testing Market Report:
The European market is projected to rise from USD 0.85 billion in 2023 to USD 1.68 billion by 2033. Strong regulatory support for innovative testing methods and increasing prevalence of heart diseases are primary drivers of growth in this region.Asia Pacific Cardiac Marker Testing Market Report:
The Asia Pacific region is poised for significant growth, with the market size expected to expand from USD 0.42 billion in 2023 to approximately USD 0.82 billion by 2033. This growth is driven by an increase in CVD cases, improvements in healthcare infrastructure, and rising public awareness regarding heart health.North America Cardiac Marker Testing Market Report:
North America remains the largest market for cardiac marker testing, anticipated to grow from USD 0.89 billion in 2023 to USD 1.74 billion by 2033, reflecting a CAGR that stems from high healthcare expenditure, advanced healthcare facilities, and rapid adoption of new technologies.South America Cardiac Marker Testing Market Report:
In South America, the cardiac marker testing market is expected to grow from USD 0.09 billion in 2023 to USD 0.17 billion by 2033. Factors contributing to this growth include an increasing focus on chronic disease management and investment in healthcare systems to improve testing access.Middle East & Africa Cardiac Marker Testing Market Report:
In the Middle East and Africa, the market is expected to grow from USD 0.26 billion in 2023 to USD 0.51 billion by 2033. The rising incidence of cardiovascular diseases and improvements in medical facilities are pivotal in expanding the market.Tell us your focus area and get a customized research report.
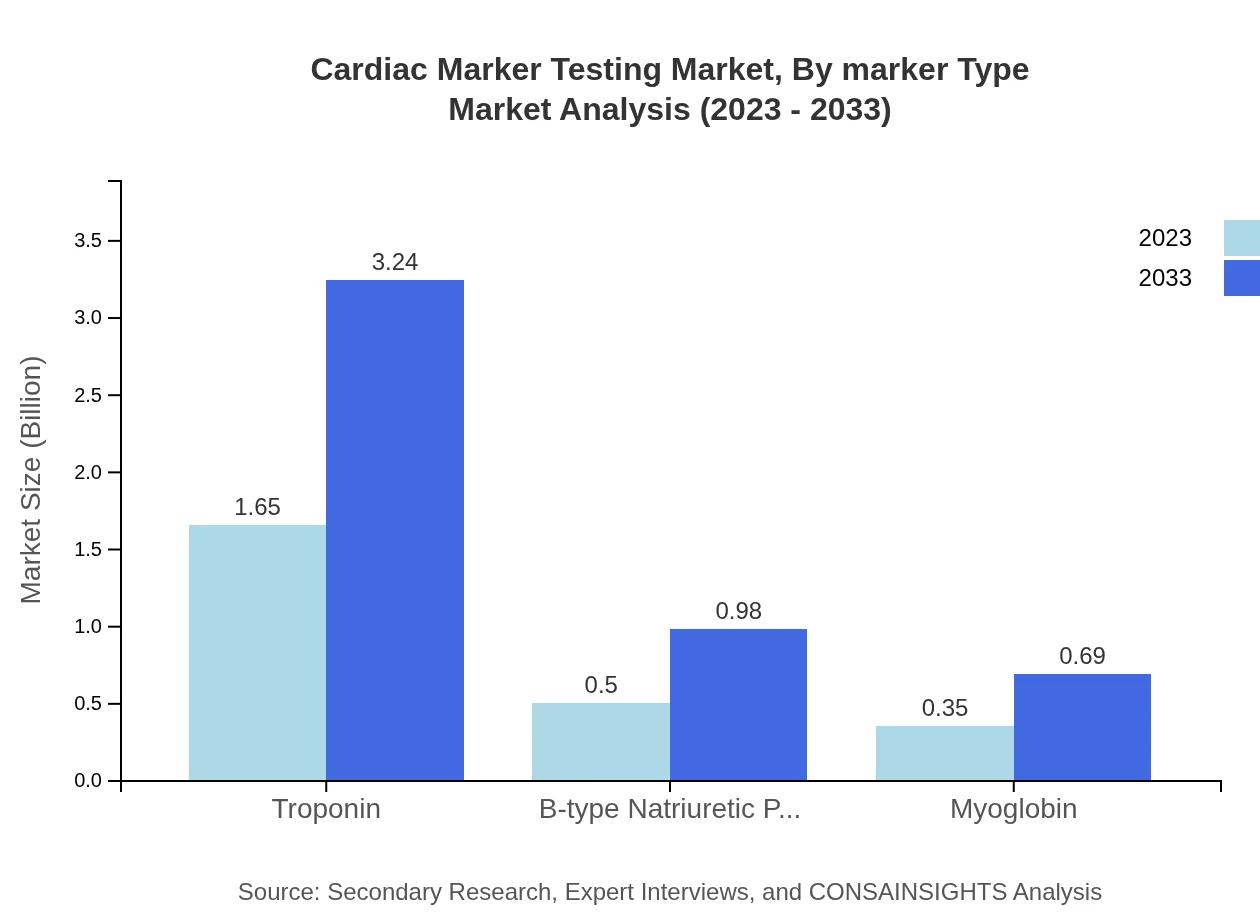
Cardiac Marker Testing Market Analysis By Marker Type
Among marker types, Troponin is expected to dominate, projected to increase from USD 1.65 billion in 2023 to USD 3.24 billion by 2033, with a consistent market share of 66.02%. B-type Natriuretic Peptide (BNP) and Myoglobin are also significant players, capturing 20% and 13.98% of the respective markets in 2023.
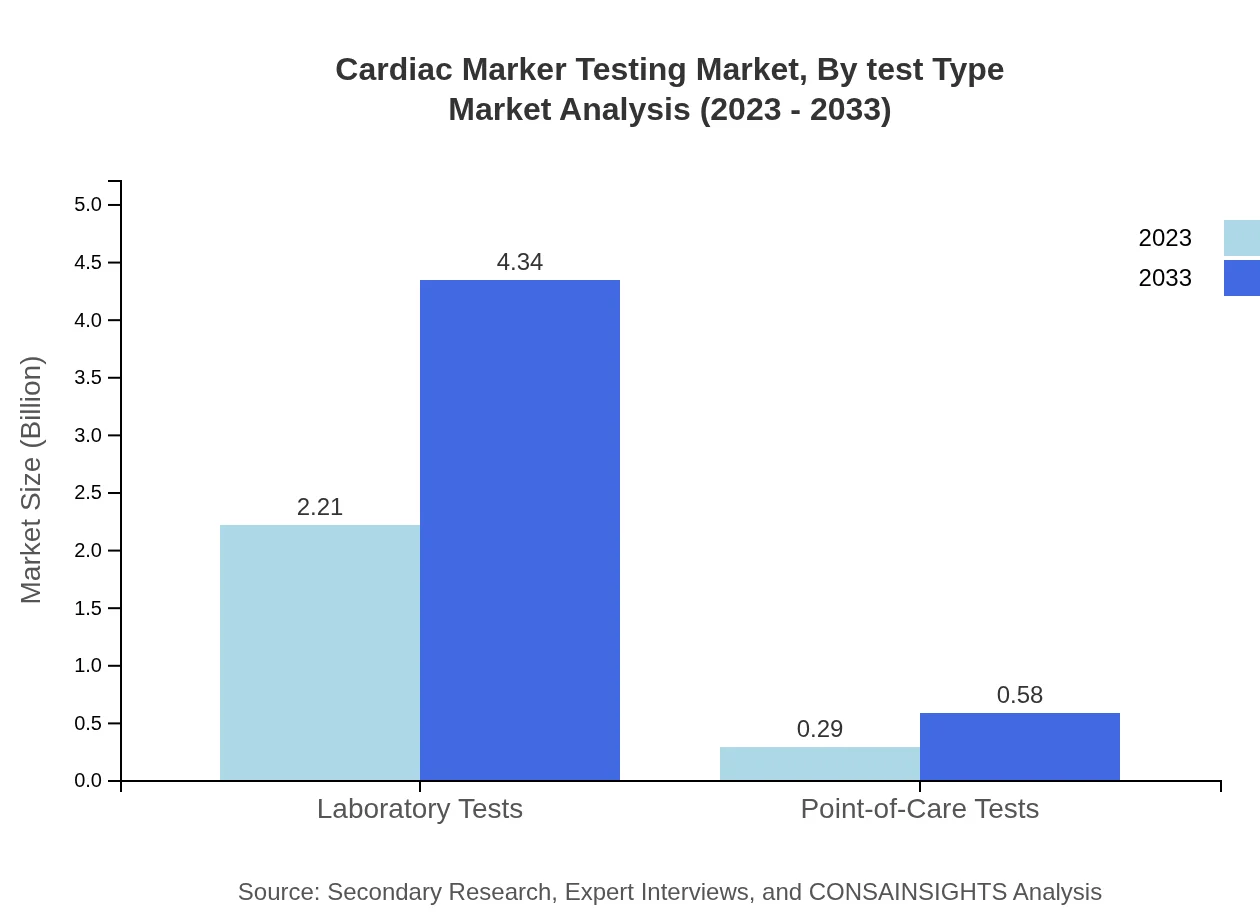
Cardiac Marker Testing Market Analysis By Test Type
Laboratory tests are the predominant test type, valued at USD 2.21 billion in 2023 with a projected growth to USD 4.34 billion by 2033, holding 88.28% of the market share. Point-of-care tests, although smaller, are vital for rapid diagnostics, expected to rise significantly from USD 0.29 billion to USD 0.58 billion.
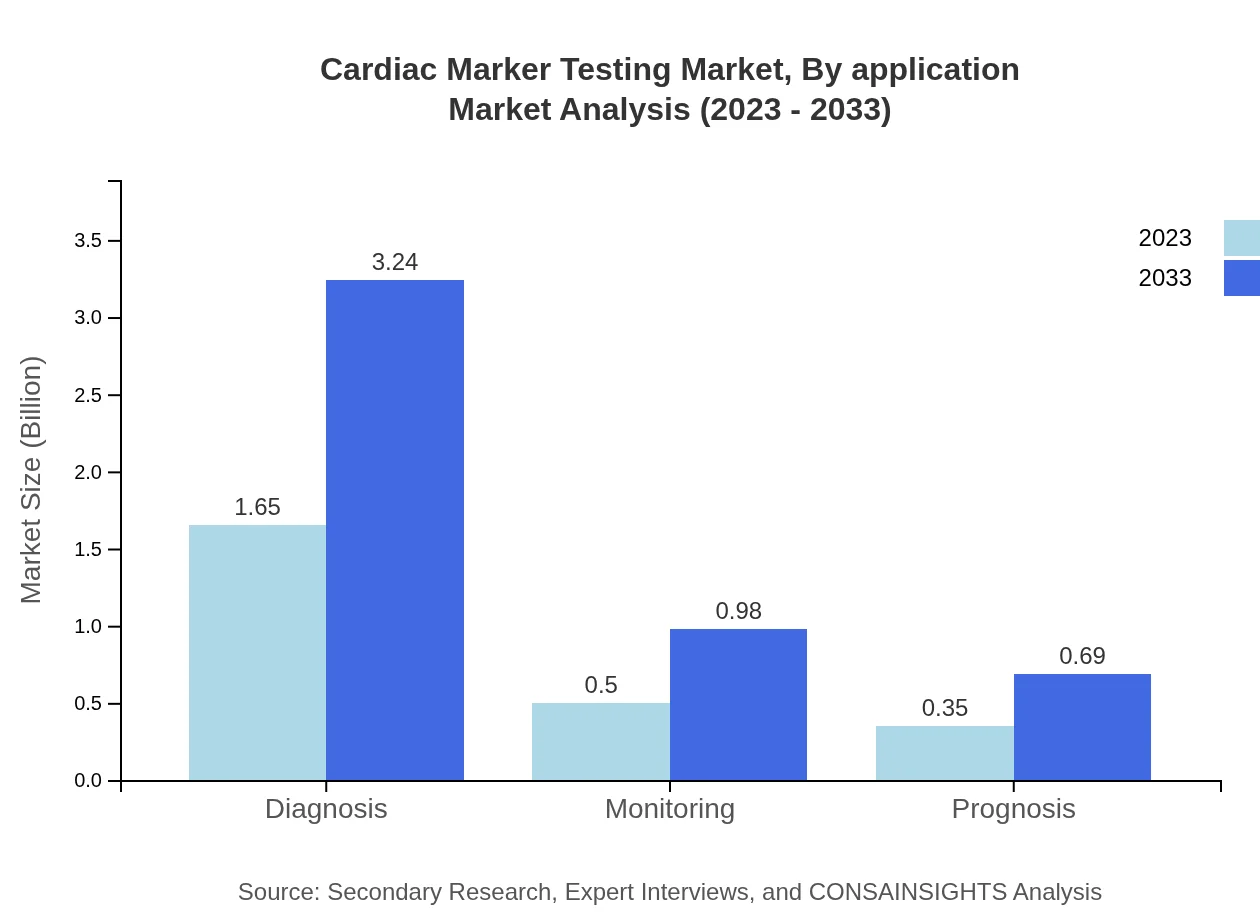
Cardiac Marker Testing Market Analysis By Application
The diagnosis segment leads the market, segmented at USD 1.65 billion in 2023 to USD 3.24 billion by 2033, representing 66.02% share. Monitoring applications, while smaller, are anticipated to maintain a 20% market share, emphasizing the importance of ongoing patient assessment.
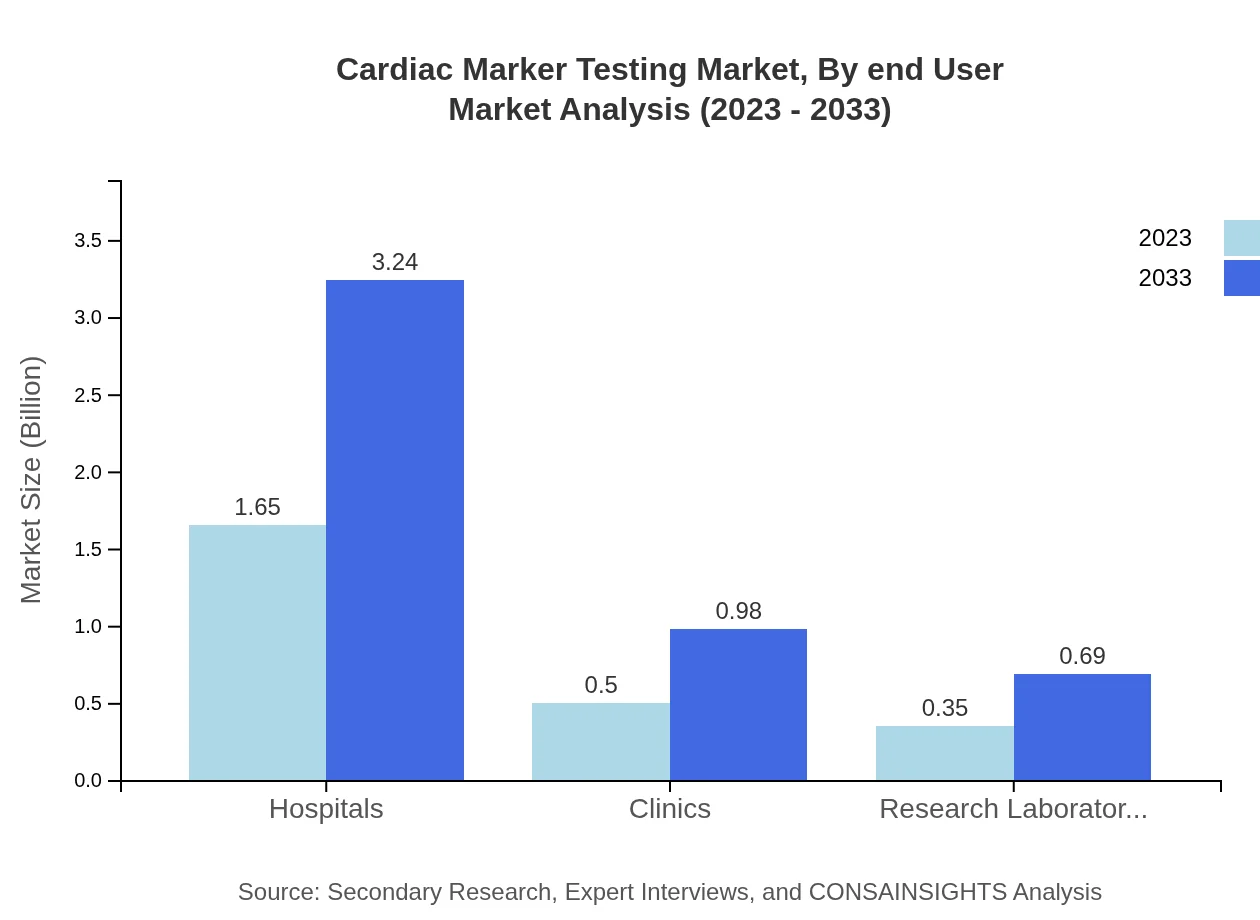
Cardiac Marker Testing Market Analysis By End User
Hospitals are the predominant end-users, holding a market size of USD 1.65 billion in 2023, with an increase to USD 3.24 billion by 2033 and a market share of 66.02%. Clinics and research laboratories are also important, accounting for 20% and 13.98% of the market share, respectively.
Cardiac Marker Testing Market Analysis By Region
The regional analysis highlights North America as the largest market, followed by Europe and the Asia Pacific, each experiencing significant growth driven by technological improvements and increased awareness about cardiovascular health.
Cardiac Marker Testing Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cardiac Marker Testing Industry
Roche Diagnostics:
Roche Diagnostics is a leader in laboratory diagnostics, particularly in cardiac care. They provide a comprehensive range of innovative cardiac marker tests that enhance early diagnosis and treatment of heart diseases.Abbott Laboratories:
Abbott Laboratories specializes in medical devices and diagnostics, offering a broad spectrum of cardiac markers that are widely utilized in both laboratory and point-of-care settings, significantly improving patient outcomes.Siemens Healthineers:
Siemens Healthineers provides advanced imaging and laboratory diagnostics solutions, including cardiac marker testing technologies that assist clinicians in making timely clinical decisions.Beckman Coulter:
Beckman Coulter, part of Danaher Corporation, focuses on laboratory diagnostics, delivering innovative cardiac testing solutions that enable accurate assessment of cardiovascular diseases.Thermo Fisher Scientific:
Thermo Fisher Scientific is known for its diagnostics and laboratory equipment. Its cardiac testing solutions are integral to advancements in cardiovascular diagnostics.We're grateful to work with incredible clients.









FAQs
What is the market size of cardiac Marker Testing?
The global cardiac marker testing market is valued at approximately $2.5 billion in 2023 and is projected to grow at a CAGR of 6.8%, reaching significant growth by 2033.
What are the key market players or companies in the cardiac Marker Testing industry?
Key players in the cardiac marker testing market include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and Thermo Fisher Scientific, among others, who drive innovation and product development in this field.
What are the primary factors driving the growth in the cardiac marker testing industry?
Major growth drivers include rising cardiovascular diseases, technological advancements in test accuracy, increasing awareness regarding cardiac health, and a growing geriatric population worldwide.
Which region is the fastest Growing in the cardiac marker testing?
North America is currently the largest market, while Europe is experiencing substantial growth. Asia-Pacific is expected to be the fastest-growing region, expanding from $0.27 billion in 2023 to $0.53 billion by 2033.
Does ConsaInsights provide customized market report data for the cardiac Marker Testing industry?
Yes, ConsaInsights offers tailored market research reports, catering to specific client needs and providing detailed insights into market segments and trends.
What deliverables can I expect from this market research project?
Deliverables include comprehensive market analysis, trend forecasting, competitive landscape assessments, regional market insights, and detailed segment performance data.
What are the market trends of cardiac Marker Testing?
Current trends in the cardiac marker testing market include increasing adoption of point-of-care testing, rising demand for rapid testing solutions, and advancements in biomarker technology, enhancing diagnostic capabilities.