Cardiac Safety Services Market Report
Published Date: 31 January 2026 | Report Code: cardiac-safety-services
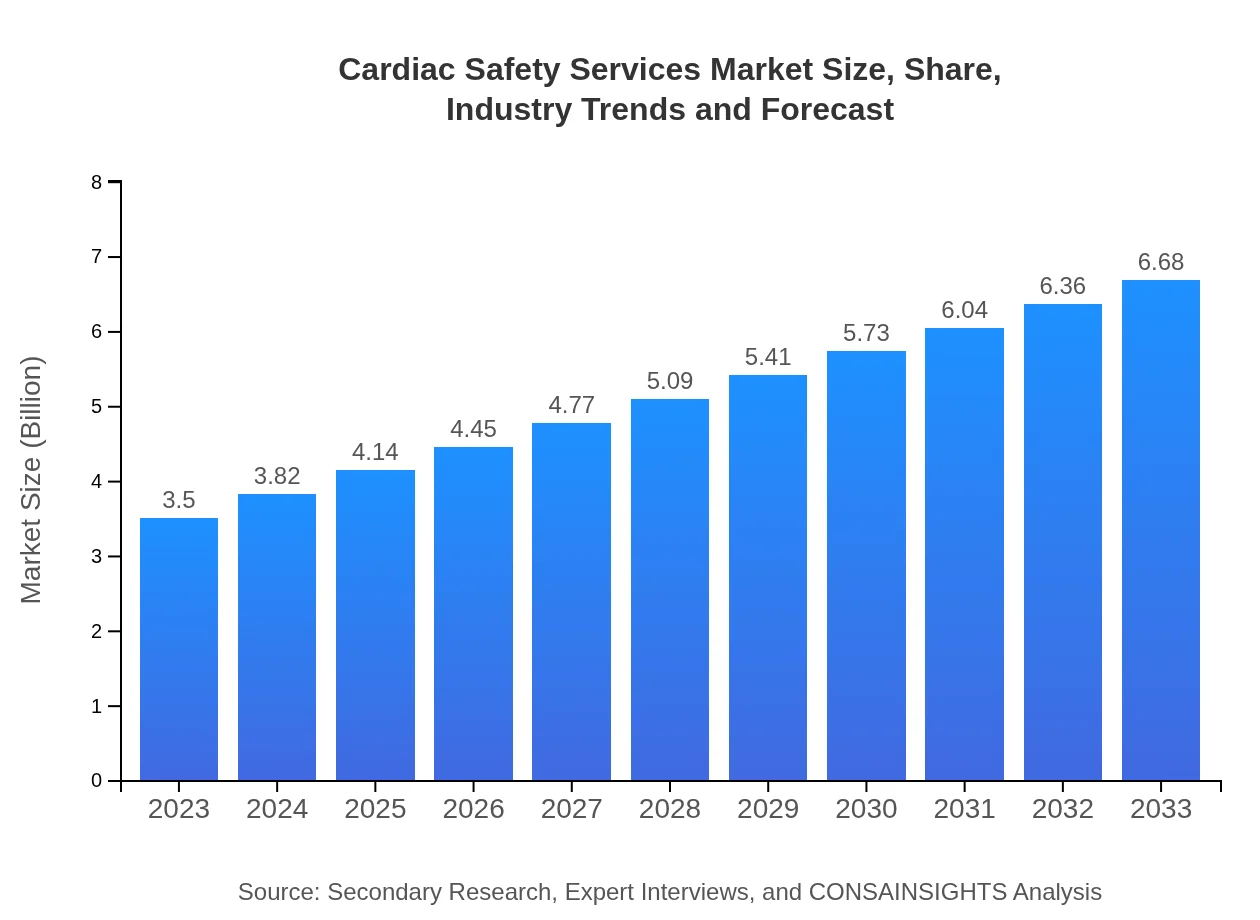
Cardiac Safety Services Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the cardiac safety services market, highlighting comprehensive insights, forecasts from 2023 to 2033, market trends, segment performance, regional analysis, and key players within the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
| 2023 Market Size | $3.50 Billion |
| CAGR (2023-2033) | 6.5% |
| 2033 Market Size | $6.68 Billion |
| Top Companies | Icon plc, PPD, Inc., Charles River Laboratories, QuintilesIMS |
| Last Modified Date | 31 January 2026 |
Cardiac Safety Services Market Overview
Customize Cardiac Safety Services Market Report market research report
- ✔ Get in-depth analysis of Cardiac Safety Services market size, growth, and forecasts.
- ✔ Understand Cardiac Safety Services's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cardiac Safety Services
What is the Market Size & CAGR of Cardiac Safety Services market in 2023?
Cardiac Safety Services Industry Analysis
Cardiac Safety Services Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cardiac Safety Services Market Analysis Report by Region
Europe Cardiac Safety Services Market Report:
The European market is expected to grow from $1.14 billion in 2023 to $2.18 billion by 2033. Key drivers include an aging population, increasing chronic disease prevalence, and robust research frameworks that emphasize cardiac safety.Asia Pacific Cardiac Safety Services Market Report:
In 2023, the cardiac safety services market in the Asia-Pacific region is valued at $0.66 billion, with expectations to grow to $1.26 billion by 2033. The growth in this region is driven by increasing investments in healthcare infrastructure, rising awareness about cardiac health, and the growing number of clinical trials, particularly in countries like China and India.North America Cardiac Safety Services Market Report:
North America currently dominates the cardiac safety services market, with a valuation of $1.19 billion in 2023 and projected growth to $2.27 billion by 2033. The region's lead is attributed to well-established healthcare systems, high levels of investment in clinical research, and stringent regulatory adherence.South America Cardiac Safety Services Market Report:
The South American market for cardiac safety services is projected to grow from $0.13 billion in 2023 to $0.24 billion by 2033. This growth is fueled by an increase in cardiovascular diseases and a rise in research activities to improve drug safety standards in the region.Middle East & Africa Cardiac Safety Services Market Report:
In the Middle East and Africa, the market for cardiac safety services is expected to rise from $0.39 billion in 2023 to $0.74 billion by 2033, primarily driven by enhancements in healthcare policies and increasing investments in clinical trial initiatives.Tell us your focus area and get a customized research report.
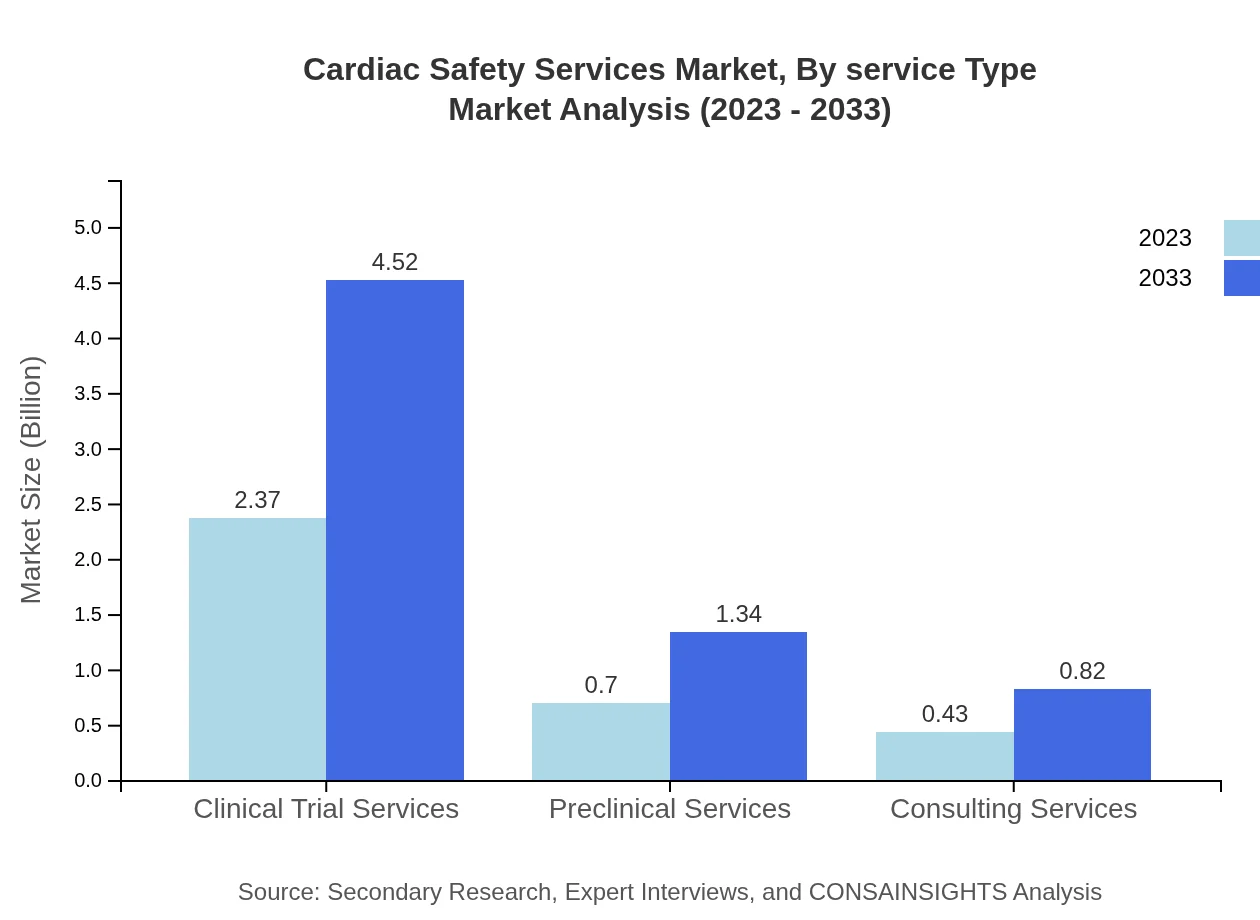
Cardiac Safety Services Market Analysis By Service Type
This market segment includes clinical trial services and preclinical services, contributing a combined market size of $2.37 billion in 2023, expected to reach $4.52 billion by 2033. These services are primarily utilized by pharmaceutical companies and biotechnology firms to assess cardiac risks associated with new drug applications and are critical in preventing adverse cardiac events during clinical trials.
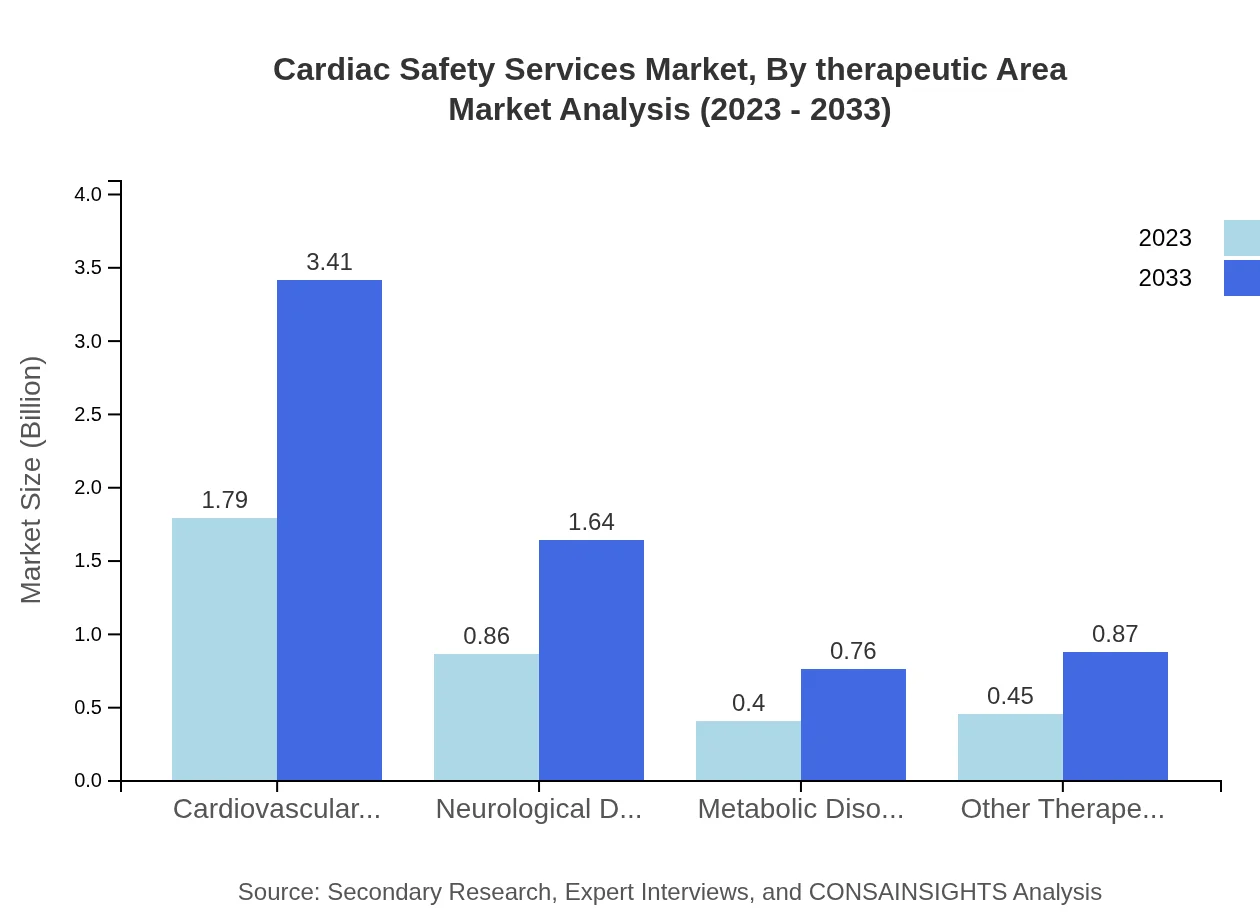
Cardiac Safety Services Market Analysis By Therapeutic Area
The market is segmented by therapeutic areas such as cardiovascular disorders, neurological disorders, and metabolic disorders. Cardiovascular disorders represented a significant share of $1.79 billion in 2023, growing to $3.41 billion by 2033, which underscores the emphasis on cardiac safety in related clinical assessments across the therapeutics landscape.
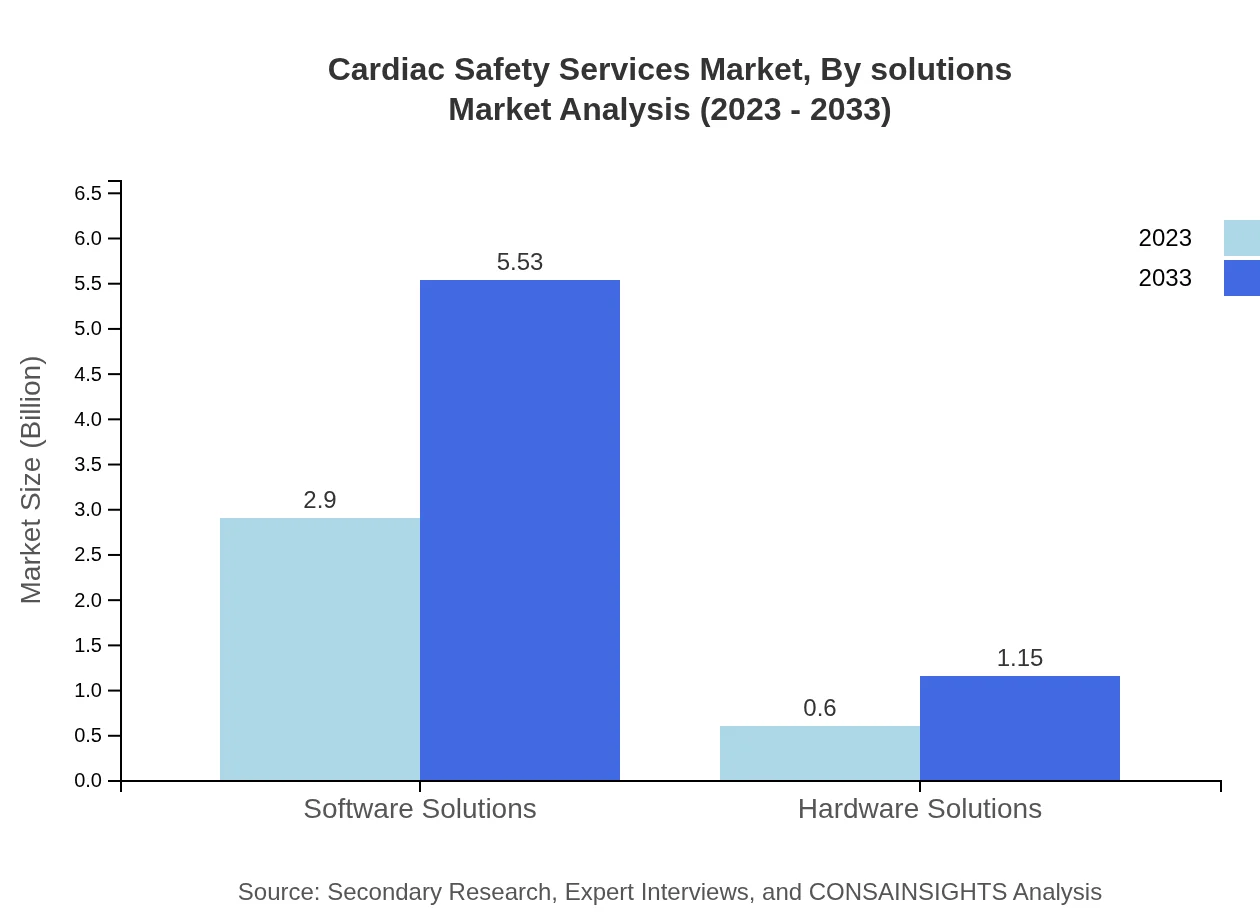
Cardiac Safety Services Market Analysis By Solutions
The market is divided into software solutions and hardware solutions. Software solutions accounted for a market size of $2.90 billion in 2023, expected to expand to $5.53 billion by 2033, attributed to advancements in digital health tools for ECG monitoring and real-time data analysis.
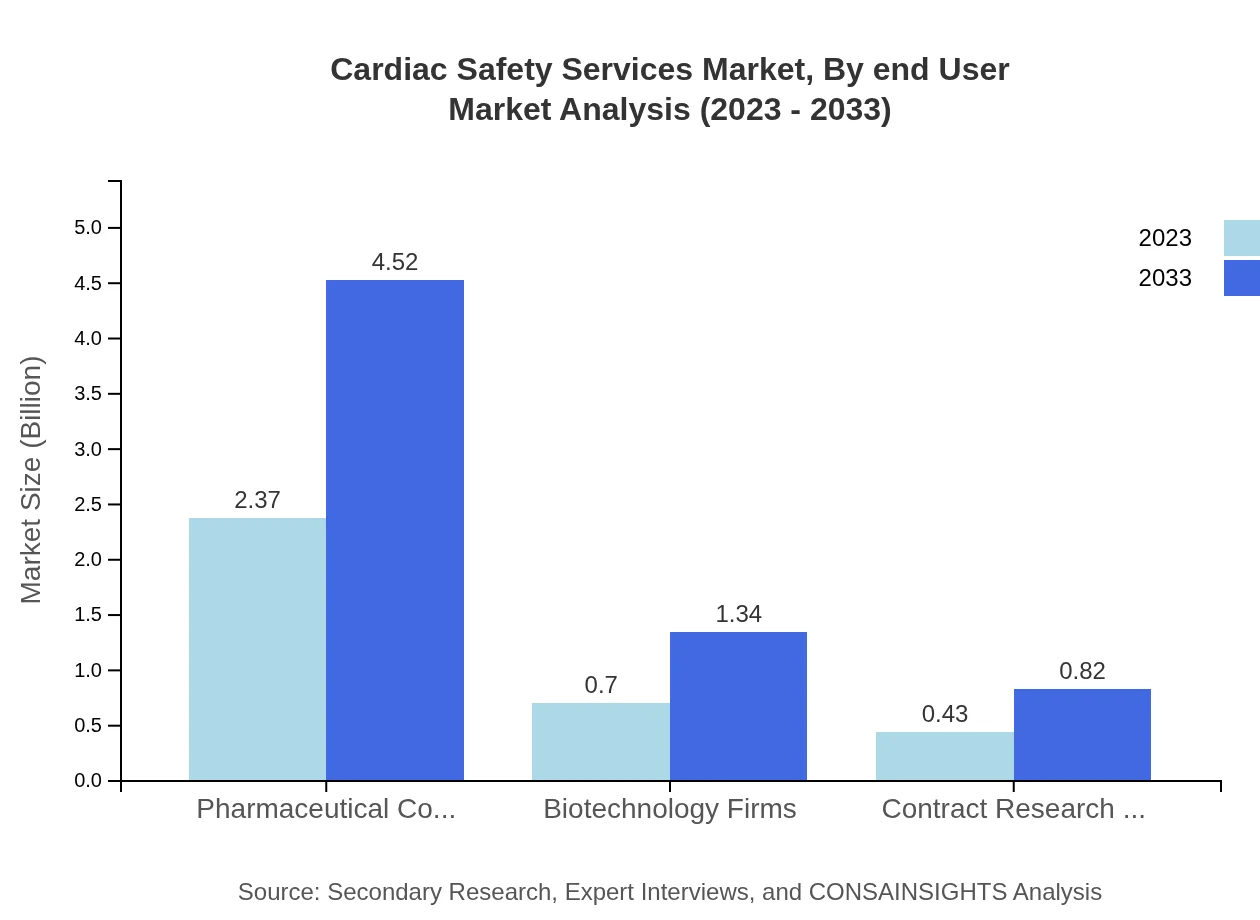
Cardiac Safety Services Market Analysis By End User
End users primarily include pharmaceutical companies, biotechnology firms, and contract research organizations, with pharmaceutical companies leading at a market size of $2.37 billion and maintaining a steady market share of 67.62%. This indicates a strong reliance on cardiac safety services in drug development processes.
Cardiac Safety Services Market Analysis By Geographic Location
Regional analysis shows North America dominating the market due to its advanced research infrastructure and significant investment in clinical trials. Europe follows closely, with substantial growth driven by regulatory pressures and patient safety requirements. Asia-Pacific is emerging as a key market as more trials are conducted seeking diverse participant pools.
Cardiac Safety Services Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cardiac Safety Services Industry
Icon plc:
Icon plc is a global provider of outsourced drug and device development services. They have extensive capabilities in cardiac safety services, leveraging advanced data analytics and expertise to streamline clinical trials.PPD, Inc.:
PPD, Inc. offers comprehensive services in drug development and lifecycle management, specializing in cardiac safety assessments and risk management for clinical trials.Charles River Laboratories:
Charles River provides a wide range of preclinical and clinical laboratory services for the biotech and pharmaceutical industries, emphasizing the importance of cardiac safety in their offerings.QuintilesIMS:
QuintilesIMS delivers integrated biopharmaceutical services and has a strong focus on cardiac safety evaluations in their clinical research methodologies.We're grateful to work with incredible clients.









FAQs
What is the market size of cardiac Safety Services?
The global Cardiac Safety Services market is estimated to reach approximately $3.5 billion by 2033, growing at a CAGR of 6.5% from the current size in 2023. This growth reflects the increasing focus on cardiovascular research and patient safety.
What are the key market players or companies in this cardiac Safety Services industry?
Key players in the cardiac safety services market include major pharmaceutical companies, biotechnology firms, and contract research organizations that specialize in cardiac safety monitoring and clinical trial management.
What are the primary factors driving the growth in the cardiac Safety Services industry?
Growth in the cardiac safety services industry is driven by an aging population, increasing prevalence of cardiovascular diseases, advancements in cardiac monitoring technology, and stringent regulatory requirements for drug safety.
Which region is the fastest Growing in the cardiac safety services?
The fastest-growing region for cardiac safety services is North America, projected to grow from $1.19 billion in 2023 to $2.27 billion by 2033. Europe follows closely with substantial growth driven by increasing clinical trials.
Does ConsaInsights provide customized market report data for the cardiac Safety Services industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the cardiac safety services industry, allowing clients to gain insights focused on their market segment or geographical interest.
What deliverables can I expect from this cardiac Safety Services market research project?
Deliverables from the cardiac safety services market research project include comprehensive market analysis, detailed segment data, regional insights, competitive landscape, and growth forecasts.
What are the market trends of cardiac safety services?
Key market trends include enhanced integration of software solutions for cardiac monitoring, growth of clinical trial services, and increasing investments in preclinical research to ensure comprehensive cardiac safety evaluations.