Carpal Tunnel Release Systems Market Report
Published Date: 31 January 2026 | Report Code: carpal-tunnel-release-systems
Carpal Tunnel Release Systems Market Size, Share, Industry Trends and Forecast to 2033
This market report provides comprehensive insights into the Carpal Tunnel Release Systems industry, covering market sizes, growth forecasts, segmentation, regional analysis, technological advancements, and key market participants for the forecast period from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
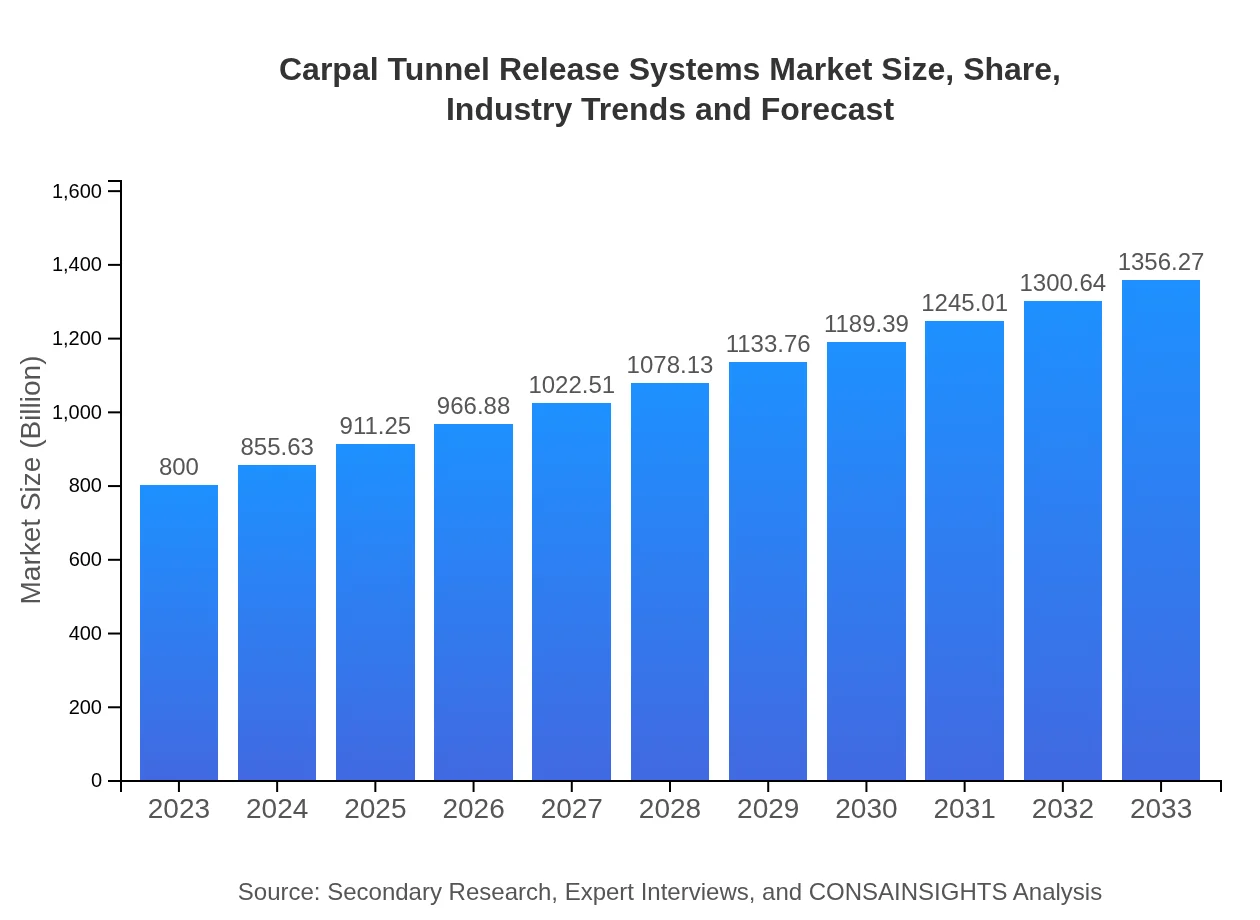
| 2023 Market Size | $800.00 Million |
| CAGR (2023-2033) | 5.3% |
| 2033 Market Size | $1356.27 Million |
| Top Companies | Medtronic , Stryker Corporation, Smith & Nephew, Zimmer Biomet |
| Last Modified Date | 31 January 2026 |
Carpal Tunnel Release Systems Market Overview
Customize Carpal Tunnel Release Systems Market Report market research report
- ✔ Get in-depth analysis of Carpal Tunnel Release Systems market size, growth, and forecasts.
- ✔ Understand Carpal Tunnel Release Systems's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Carpal Tunnel Release Systems
What is the Market Size & CAGR of Carpal Tunnel Release Systems market in 2023-2033?
Carpal Tunnel Release Systems Industry Analysis
Carpal Tunnel Release Systems Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Carpal Tunnel Release Systems Market Analysis Report by Region
Europe Carpal Tunnel Release Systems Market Report:
The European market is also witnessing substantial growth, with projections to increase from USD 226.08 million in 2023 to USD 383.28 million by 2033. The growth can be attributed to advancements in healthcare technologies and a rising number of surgical procedures.Asia Pacific Carpal Tunnel Release Systems Market Report:
In the Asia Pacific region, the market is expected to grow from USD 150.72 million in 2023 to USD 255.52 million by 2033. Factors contributing to this growth include increasing awareness of CTS treatments, improving healthcare infrastructure, and rising disposable incomes.North America Carpal Tunnel Release Systems Market Report:
North America dominates the Carpal Tunnel Release Systems market, with an estimated value of USD 309.92 million in 2023, expected to reach USD 525.42 million by 2033. The factors contributing to this growth include advanced healthcare facilities, high patient awareness, and an aging population.South America Carpal Tunnel Release Systems Market Report:
The South American market is projected to experience growth from USD 49.12 million in 2023 to USD 83.27 million in 2033. The rising prevalence of carpal tunnel syndrome and an increase in healthcare spending are key drivers for this market.Middle East & Africa Carpal Tunnel Release Systems Market Report:
In the Middle East and Africa, the market is expected to grow from USD 64.16 million in 2023 to USD 108.77 million by 2033. The rise in healthcare initiatives and improving access to advanced medical treatments are notable drivers.Tell us your focus area and get a customized research report.
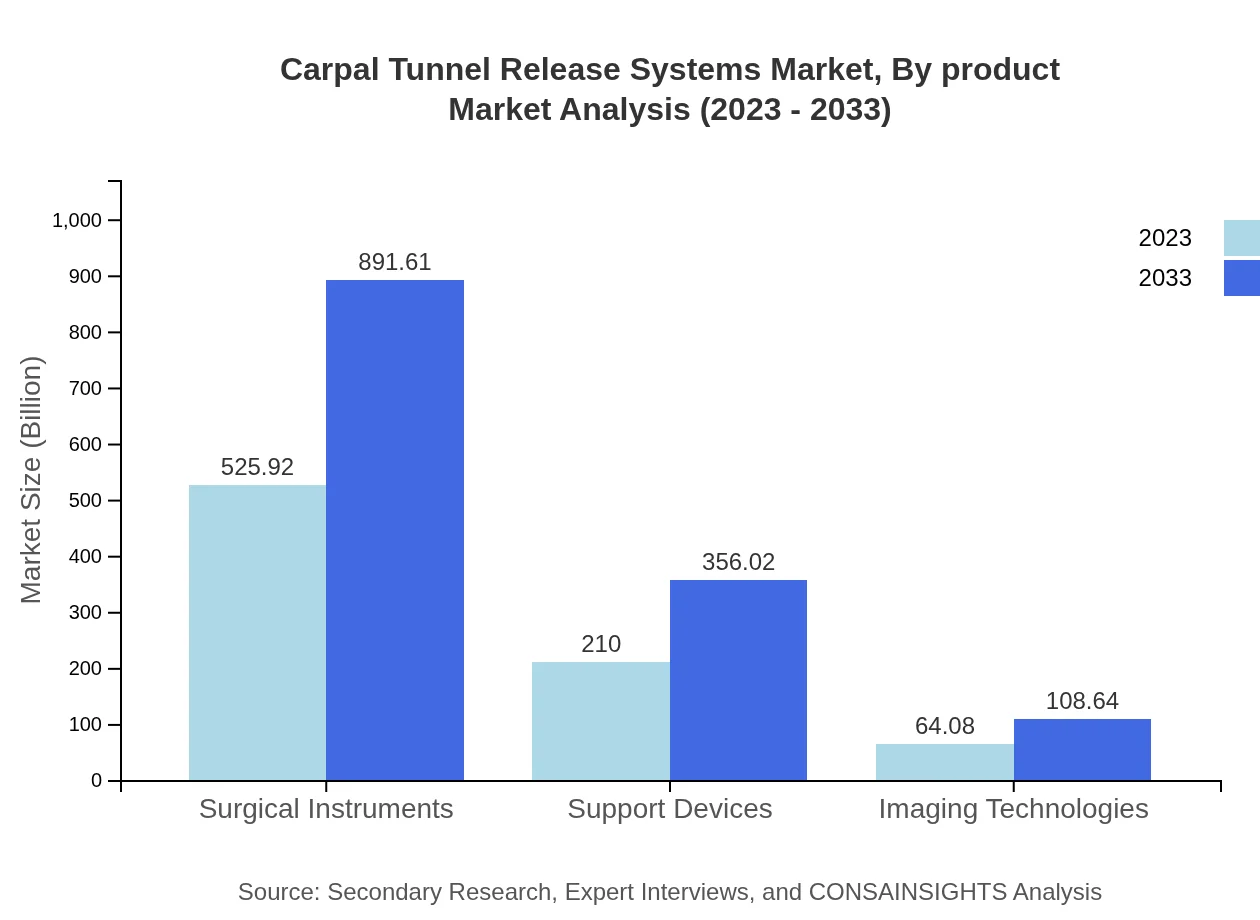
Carpal Tunnel Release Systems Market Analysis By Product
Surgical Instruments represent the largest market segment, valued at USD 525.92 million in 2023, growing to USD 891.61 million by 2033, accounting for 65.74% of the market share throughout the forecast period. Support Devices also show promising growth, projected from USD 210.00 million in 2023 to USD 356.02 million by 2033, representing 26.25% market share.
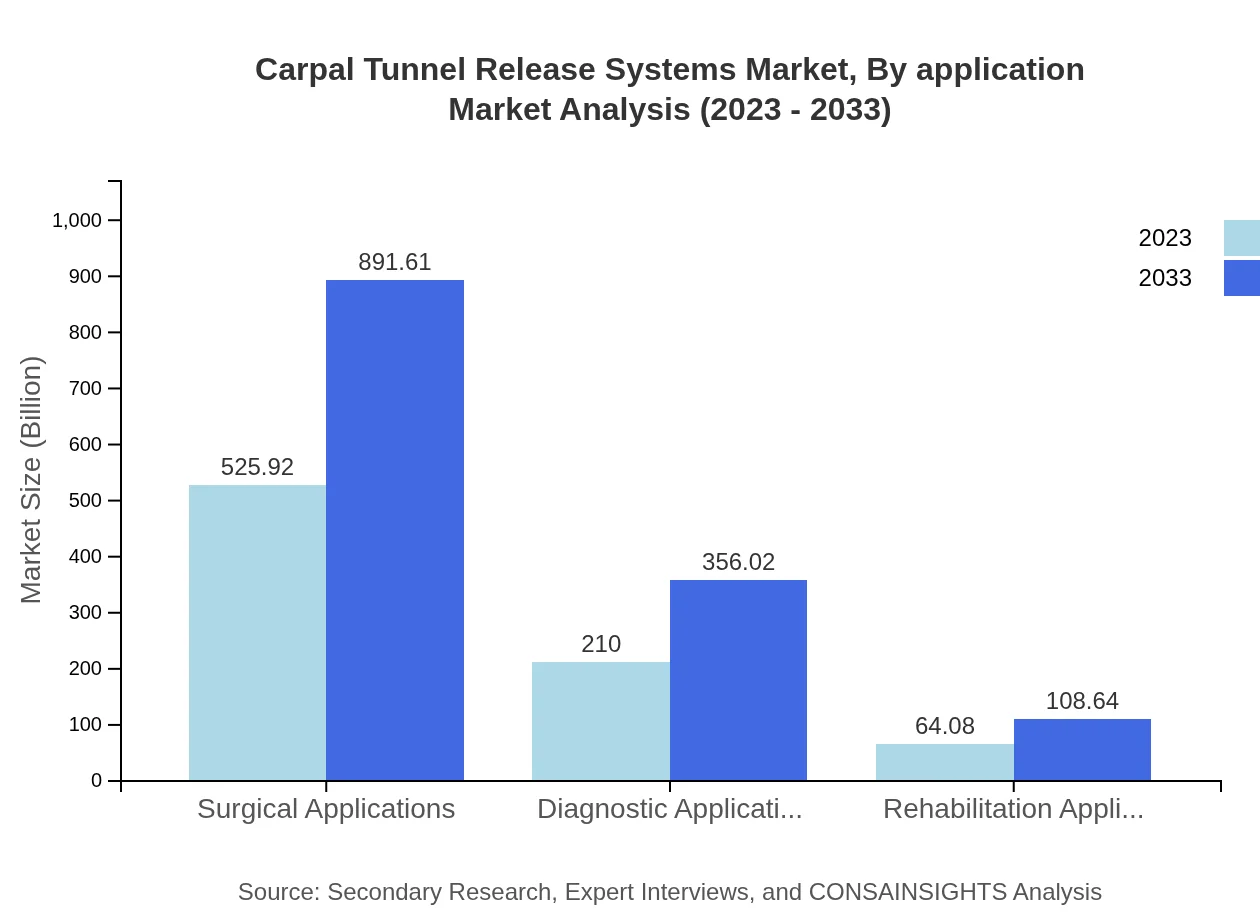
Carpal Tunnel Release Systems Market Analysis By Application
Surgical Applications dominate the application segment, similarly growing from USD 525.92 million in 2023 to USD 891.61 million by 2033. Diagnostic Applications are expected to increase from USD 210.00 million to USD 356.02 million, while Rehabilitation Applications will grow from USD 64.08 million to USD 108.64 million, emphasizing the critical role of rigorous post-operative care.
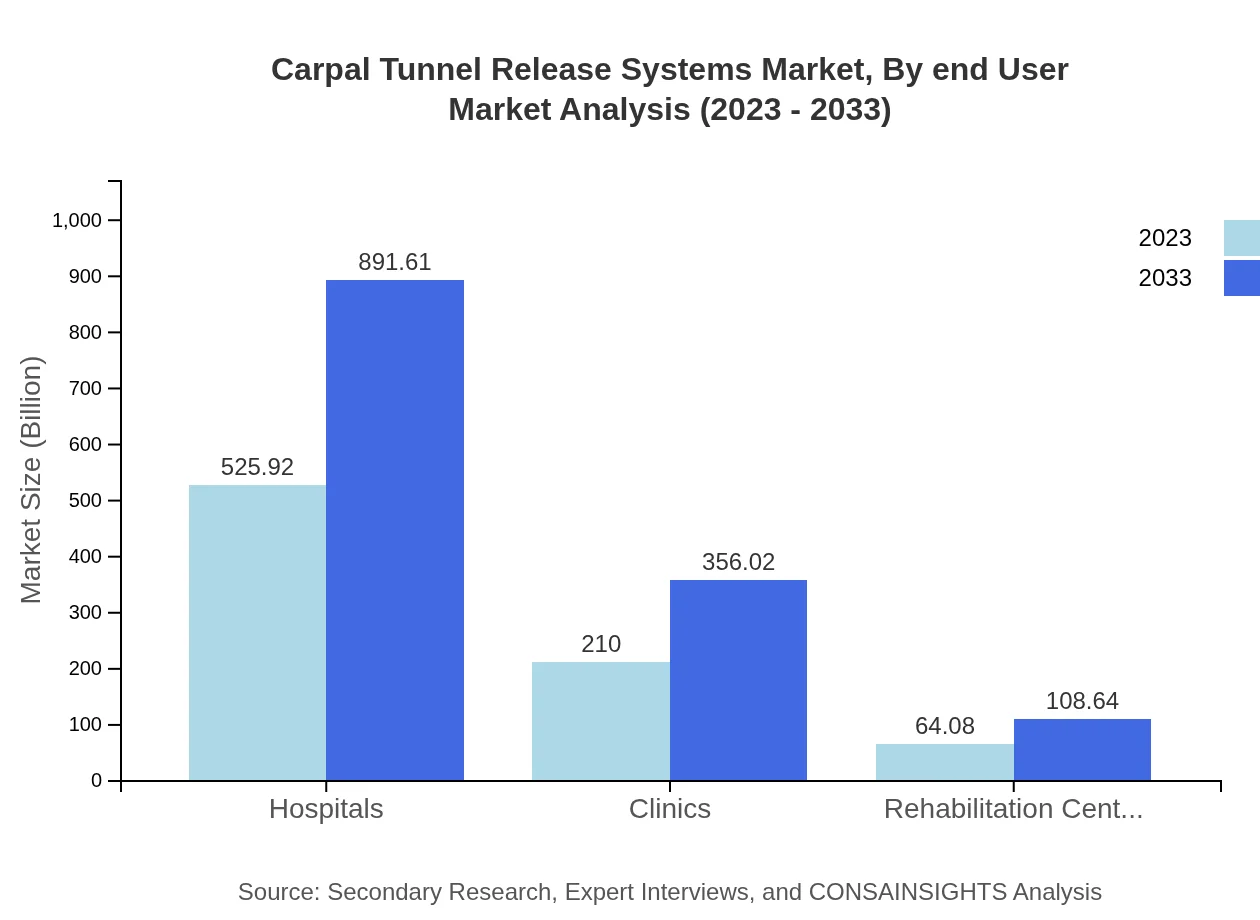
Carpal Tunnel Release Systems Market Analysis By End User
Hospitals represent the major end-user segment in the Carpal Tunnel Release Systems market, with a steady market valuation increasing significantly from USD 525.92 million in 2023 to USD 891.61 million by 2033, while Clinics are also expected to grow alongside, driven by patient accessibility.
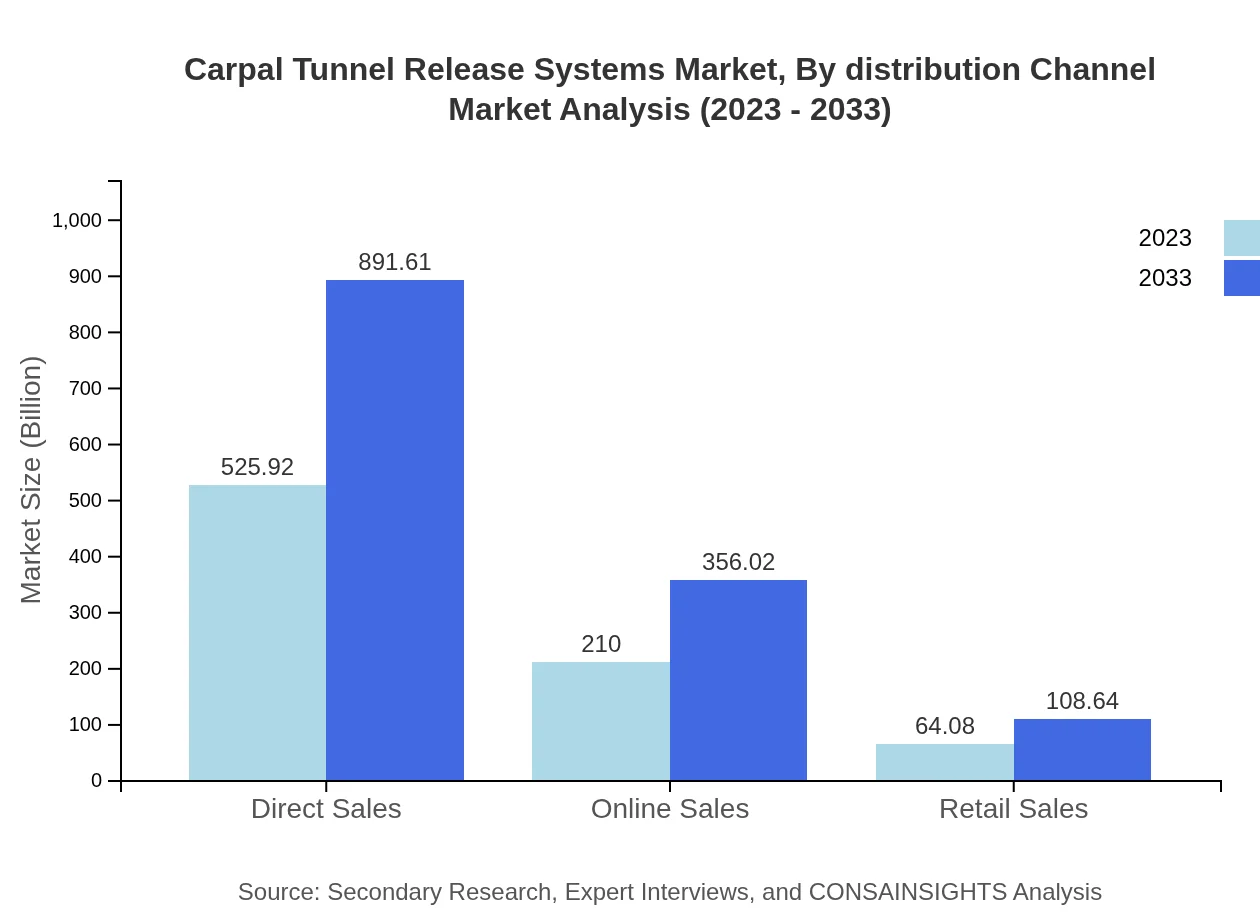
Carpal Tunnel Release Systems Market Analysis By Distribution Channel
The distribution channels show that Direct Sales hold the largest market share, projected to maintain their position from USD 525.92 million in 2023 to USD 891.61 million by 2033. Online sales are also expected to rise significantly, drawn by the increasing consumer trend towards digital purchasing.
Carpal Tunnel Release Systems Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Carpal Tunnel Release Systems Industry
Medtronic :
A leading global medical device company, Medtronic specializes in innovative surgical solutions including instruments for carpal tunnel release, aiming to enhance patient surgical outcomes.Stryker Corporation:
Known for its advanced surgical equipment and technology, Stryker actively contributes to the carpal tunnel release market through extensive product offerings and research initiatives.Smith & Nephew:
A key player in the global medical technology sector, Smith & Nephew provides various surgical instruments essential for carpal tunnel release procedures.Zimmer Biomet:
Zimmer Biomet is recognized for its orthopedic and surgical innovations, including comprehensive carpal tunnel solutions designed to improve surgical efficiency.We're grateful to work with incredible clients.









FAQs
What is the market size of carpal Tunnel Release Systems?
The global carpal tunnel release systems market is projected to reach $800 million by 2033, growing at a CAGR of 5.3% from its current size in 2023. This growth reflects increasing surgical procedures and demand for effective treatment solutions.
What are the key market players or companies in this carpal Tunnel Release Systems industry?
Key market players in the carpal tunnel release systems industry include Medtronic, Stryker Corporation, Johnson & Johnson, and Zimmer Biomet. These companies are essential in driving innovation and expanding market presence through new technologies and product offerings.
What are the primary factors driving the growth in the carpal Tunnel Release Systems industry?
The growth in the carpal tunnel release systems industry is driven by an increasing prevalence of carpal tunnel syndrome, advancements in surgical techniques, and rising awareness regarding effective treatment options among healthcare providers and patients.
Which region is the fastest Growing in the carpal Tunnel Release Systems?
North America is the fastest-growing region for carpal tunnel release systems, with a projected market size increase from $309.92 million in 2023 to $525.42 million in 2033. This growth is attributed to advanced healthcare systems and an aging population.
Does ConsaInsights provide customized market report data for the carpal Tunnel Release Systems industry?
Yes, ConsaInsights offers customized market report data tailored specifically for the carpal tunnel release systems industry, catering to unique business needs and providing insights into market trends, opportunities, and competitive analysis.
What deliverables can I expect from this carpal Tunnel Release Systems market research project?
Deliverables for carpal tunnel release systems market research include in-depth reports, market forecasts, segment analysis, competitive benchmarking, and strategic recommendations, ensuring comprehensive insights into market dynamics and growth opportunities.
What are the market trends of carpal Tunnel Release Systems?
Current market trends for carpal tunnel release systems include increasing preference for minimally invasive surgical techniques, advancements in imaging technologies, and rising use of support devices, highlighting the evolution of treatment approaches in the healthcare sector.