Catheter Securement Device Market Report
Published Date: 31 January 2026 | Report Code: catheter-securement-device
Catheter Securement Device Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Catheter Securement Device market, covering market size, growth prospects, and trends from 2023 to 2033. It offers insights into various segments, regional performances, and competitive landscapes in the industry.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
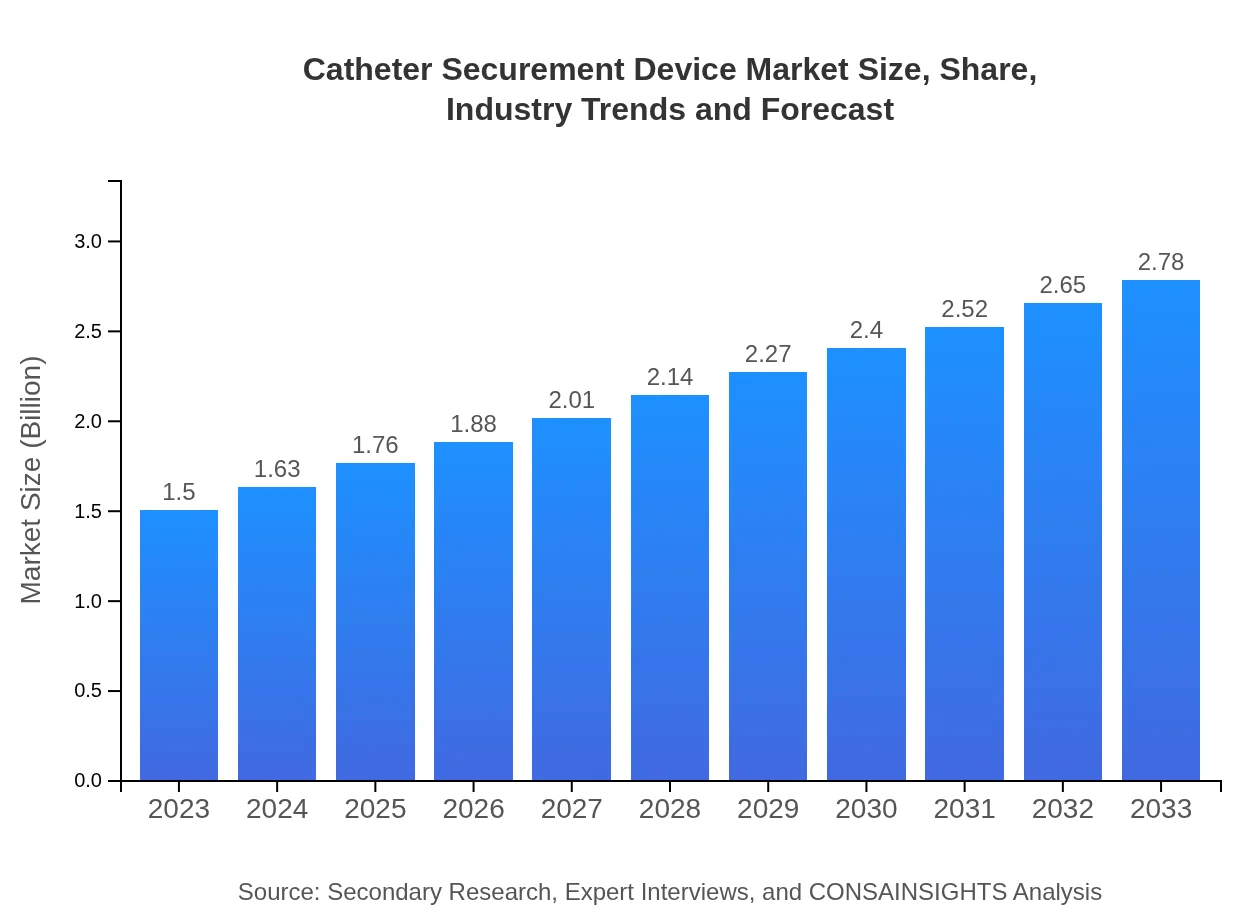
| 2023 Market Size | $1.50 Billion |
| CAGR (2023-2033) | 6.2% |
| 2033 Market Size | $2.78 Billion |
| Top Companies | Smiths Medical, B. Braun Melsungen AG, Medtronic , Teleflex Incorporated |
| Last Modified Date | 31 January 2026 |
Catheter Securement Device Market Overview
Customize Catheter Securement Device Market Report market research report
- ✔ Get in-depth analysis of Catheter Securement Device market size, growth, and forecasts.
- ✔ Understand Catheter Securement Device's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Catheter Securement Device
What is the Market Size & CAGR of the Catheter Securement Device market in 2023?
Catheter Securement Device Industry Analysis
Catheter Securement Device Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Catheter Securement Device Market Analysis Report by Region
Europe Catheter Securement Device Market Report:
In Europe, the market is valued at USD 0.42 billion in 2023, with projections of reaching USD 0.78 billion by 2033. Key factors include stringent regulatory standards and a strong preference for technologically advanced medical devices.Asia Pacific Catheter Securement Device Market Report:
In 2023, the Catheter Securement Device market in Asia Pacific is valued at USD 0.32 billion, expected to reach USD 0.59 billion by 2033. Growth in this region is driven by a rising geriatric population and increased healthcare spending focused on advanced medical technologies.North America Catheter Securement Device Market Report:
North America leads the market with a valuation of USD 0.52 billion in 2023 and is expected to grow to USD 0.97 billion by 2033. The large market size is fueled by advanced healthcare systems and emphasis on reducing catheter-related complications.South America Catheter Securement Device Market Report:
The South American market is relatively smaller, valued at USD 0.04 billion in 2023, projected to grow to USD 0.07 billion by 2033. The growth is attributed to improvements in healthcare infrastructure and increasing awareness of infection control practices.Middle East & Africa Catheter Securement Device Market Report:
The Middle East and Africa market is projected to grow from USD 0.20 billion in 2023 to USD 0.37 billion by 2033, driven by increasing healthcare expenditures and a growing awareness of patient safety.Tell us your focus area and get a customized research report.
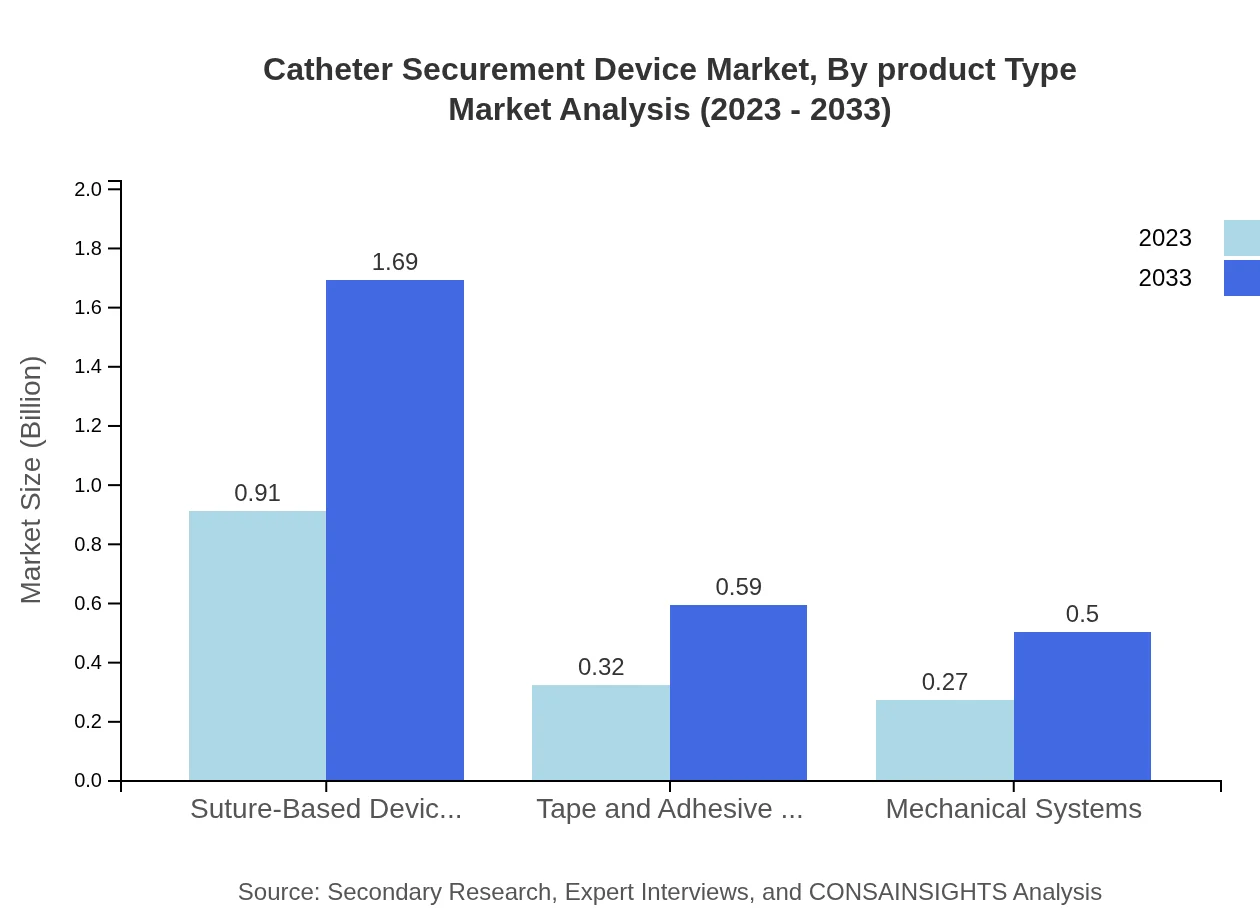
Catheter Securement Device Market Analysis By Product Type
The market performance of the Catheter Securement Device segments reveals that suture-based devices hold a significant share of the market. In 2023, their market size is approximately USD 0.91 billion and expected to grow to USD 1.69 billion by 2033. Tape and adhesive devices follow closely, with sizes of USD 0.32 billion in 2023 and projected to reach USD 0.59 billion by 2033.
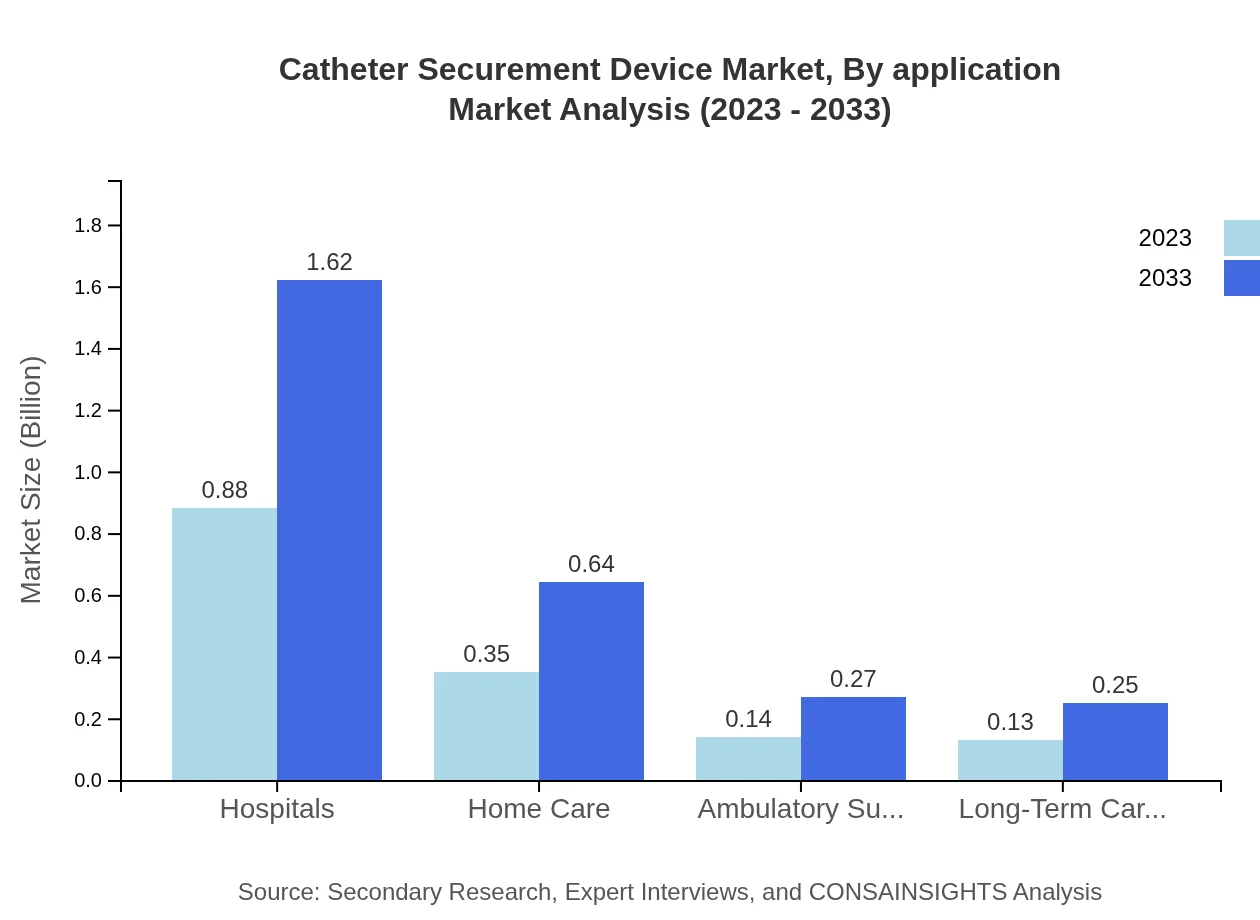
Catheter Securement Device Market Analysis By Application
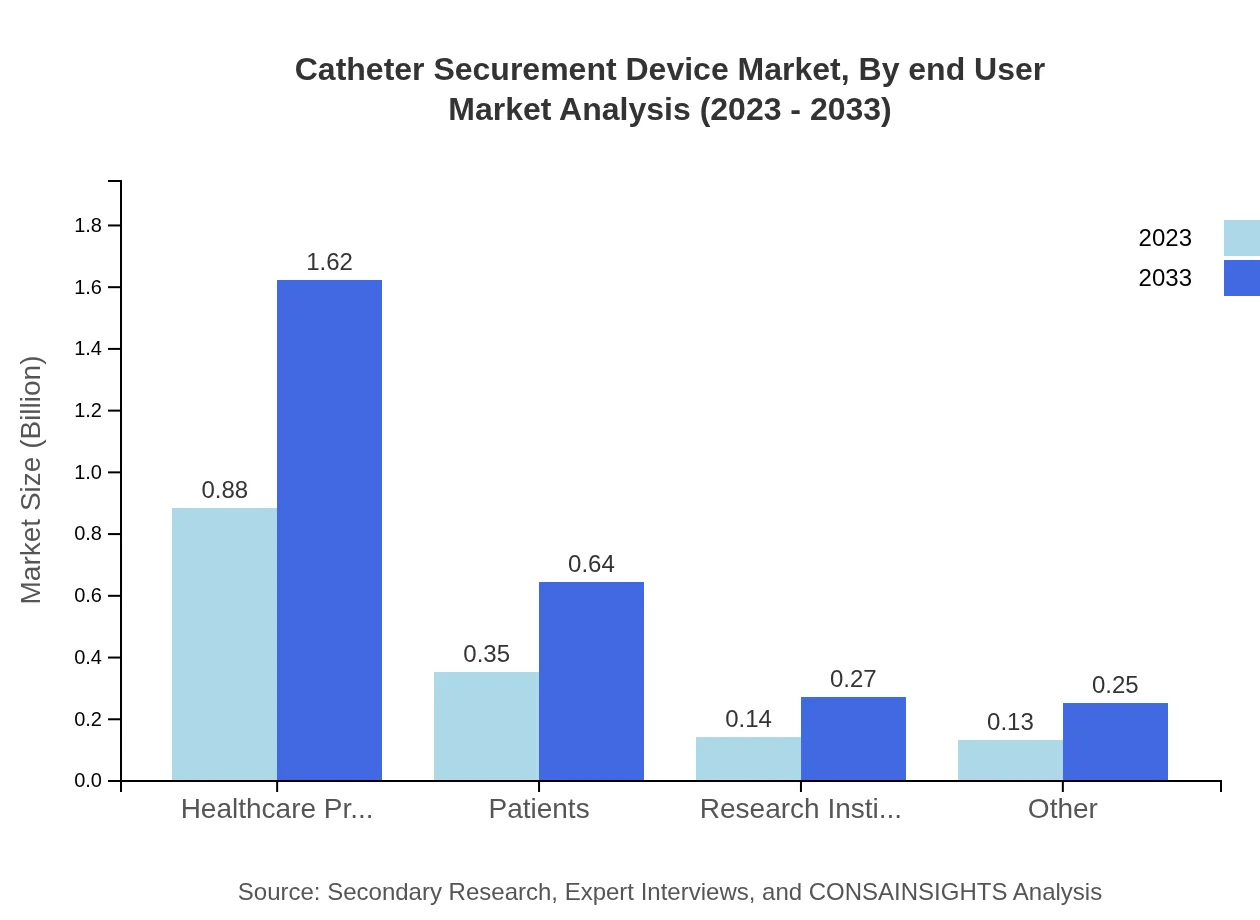
In terms of application, hospitals are the largest segment, accounting for USD 0.88 billion in 2023, with projections of USD 1.62 billion by 2033. Home care settings also present a significant opportunity, growing from USD 0.35 billion in 2023 to USD 0.64 billion in 2033.
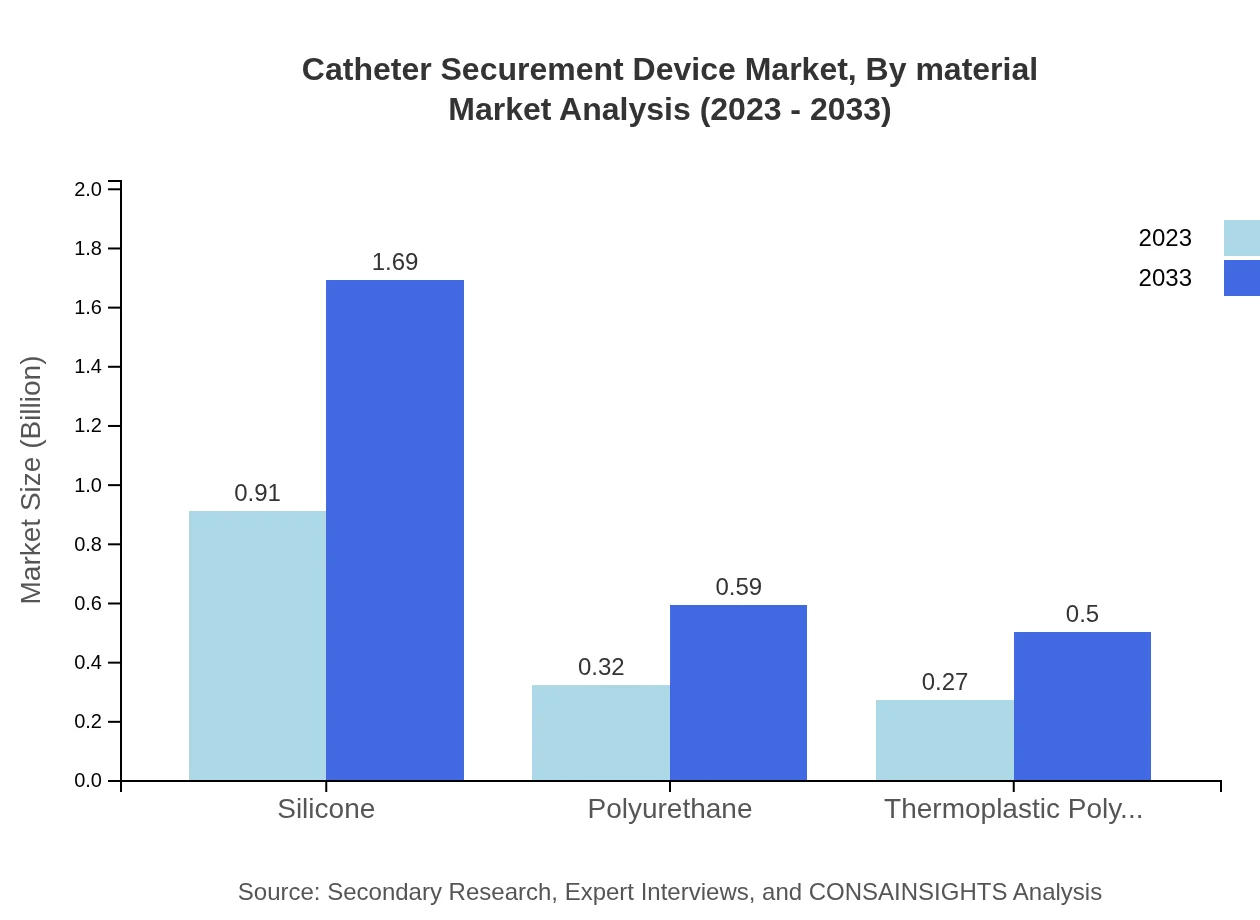
Catheter Securement Device Market Analysis By Material
Silicone materials dominate the market with a size of USD 0.91 billion in 2023, expected to reach USD 1.69 billion by 2033. Other materials, such as polyurethane and thermoplastic polyurethane, exhibit growth as demand for varied product features rises.
Catheter Securement Device Market Analysis By End User
Hospitals comprise the largest end-user segment, followed by home care facilities and long-term care facilities. Their combined market size reflects the increasing reliance on securement devices across different care settings.
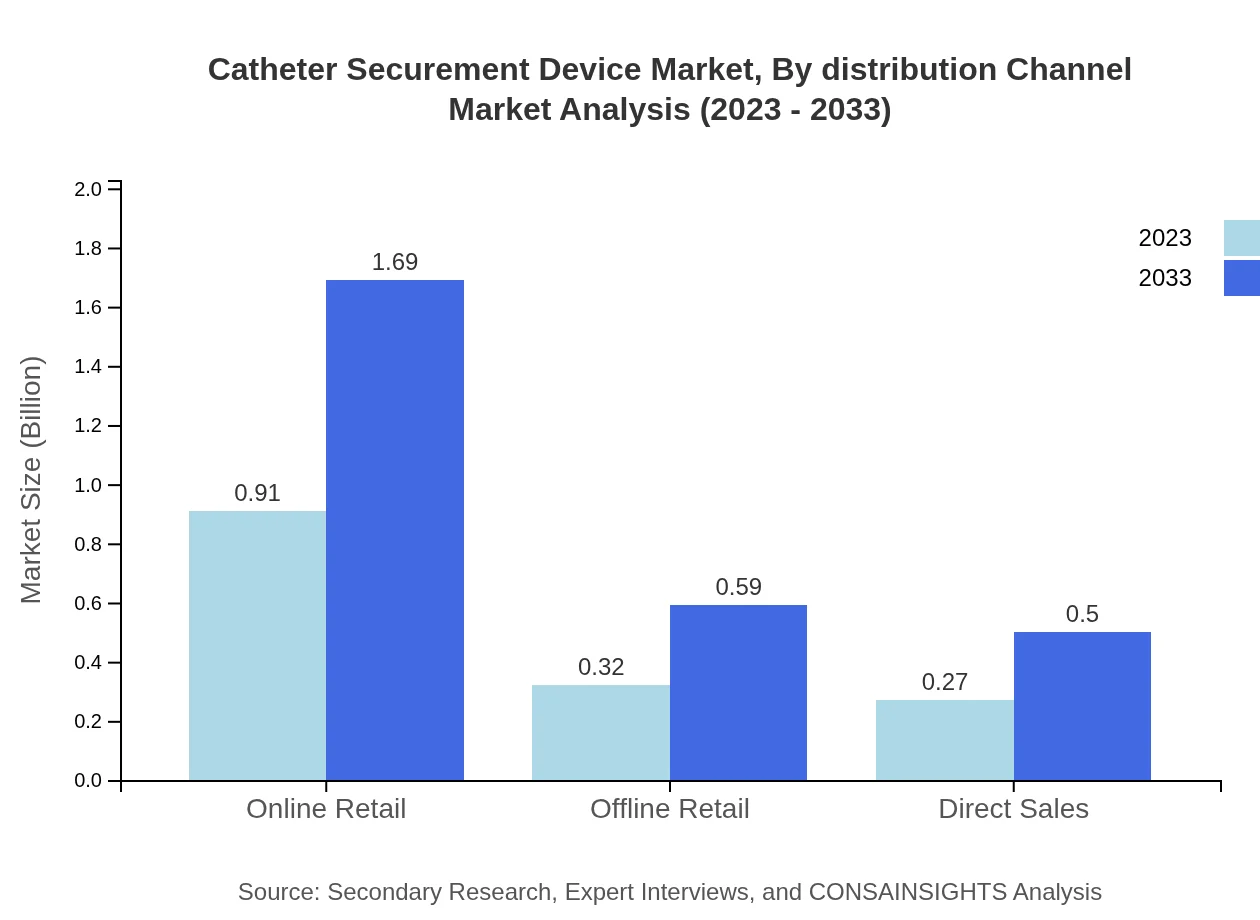
Catheter Securement Device Market Analysis By Distribution Channel
Online retail dominates the distribution channel segment, showing a market size of USD 0.91 billion in 2023, expected to grow to USD 1.69 billion by 2033. This growth underscores the shift toward e-commerce in medical supplies.
Catheter Securement Device Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Catheter Securement Device Industry
Smiths Medical:
A leading manufacturer specializing in securement solutions and innovative medical technologies.B. Braun Melsungen AG:
A global player offering a range of catheter devices and securement technologies emphasizing patient safety.Medtronic :
An international leader in medical technology providing robust catheter securement devices and comprehensive healthcare solutions.Teleflex Incorporated:
Known for its advanced catheter solutions and securement devices aimed at improving patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of catheter Securement Device?
The global catheter securement device market is valued at approximately $1.5 billion in 2023 and is projected to grow at a CAGR of 6.2%, indicating significant growth potential up to 2033.
What are the key market players or companies in this industry?
Key players in the catheter securement device market include leading medical device manufacturers and innovative startups focused on catheter securement solutions, contributing to advancements and competitive offerings in this dynamic industry.
What are the primary factors driving the growth in the catheter Securement Device industry?
Growth in the catheter securement device industry is driven by increasing healthcare expenditures, rising prevalence of chronic diseases, and advancements in medical technology, enhancing the demand for securement solutions among healthcare providers.
Which region is the fastest Growing in the catheter Securement Device market?
The fastest-growing regions in the catheter securement device market include North America, Europe, and Asia Pacific, with notable market expansions expected due to increased healthcare investments and aging populations.
Does ConsaInsights provide customized market report data for the catheter Securement Device industry?
Yes, ConsaInsights provides customized market report data tailored to specific client needs in the catheter securement device industry, offering insights based on unique business requirements and market dynamics.
What deliverables can I expect from this catheter Securement Device market research project?
Deliverables from the catheter securement device market research project include comprehensive market analysis, competitive landscape assessments, and actionable insights tailored to support strategic decision-making.
What are the market trends of catheter Securement Devices?
Key market trends in the catheter securement device sector include the adoption of innovative materials, increased focus on patient safety, and a shift towards personalized medicine strategies to enhance device usability.