Catheter Stabilization Device Catheter Securement Devices Market Report
Published Date: 31 January 2026 | Report Code: catheter-stabilization-device-catheter-securement-devices
Catheter Stabilization Device Catheter Securement Devices Market Size, Share, Industry Trends and Forecast to 2033
This comprehensive market report analyzes the Catheter Stabilization Device Catheter Securement Devices market, providing insights into market trends, size, and growth forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
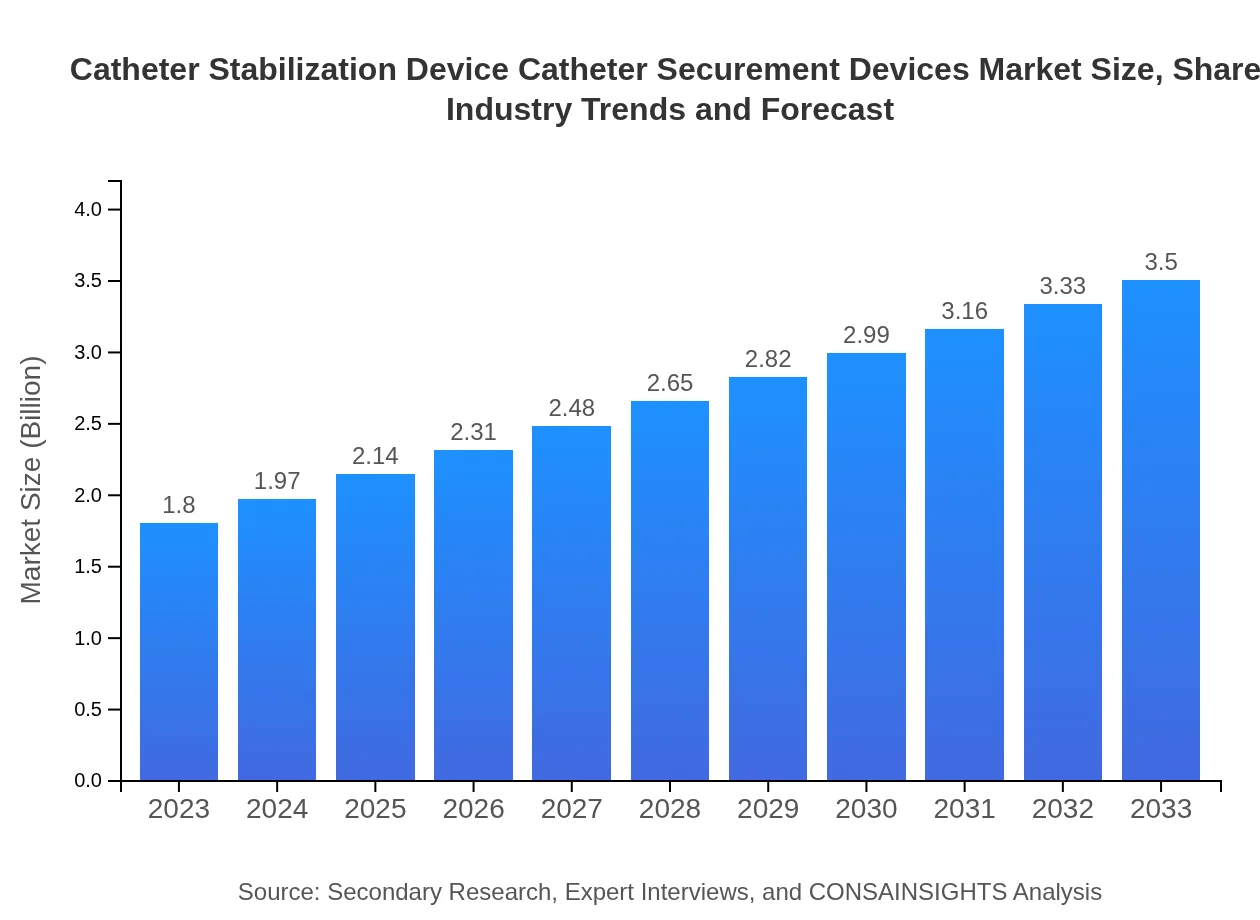
| 2023 Market Size | $1.80 Billion |
| CAGR (2023-2033) | 6.7% |
| 2033 Market Size | $3.50 Billion |
| Top Companies | Baxter International Inc., 3M Company, Smith & Nephew, Medtronic Plc, Cardinal Health |
| Last Modified Date | 31 January 2026 |
Catheter Stabilization Device Catheter Securement Devices Market Overview
Customize Catheter Stabilization Device Catheter Securement Devices Market Report market research report
- ✔ Get in-depth analysis of Catheter Stabilization Device Catheter Securement Devices market size, growth, and forecasts.
- ✔ Understand Catheter Stabilization Device Catheter Securement Devices's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Catheter Stabilization Device Catheter Securement Devices
What is the Market Size & CAGR of Catheter Stabilization Device Catheter Securement Devices market in 2023?
Catheter Stabilization Device Catheter Securement Devices Industry Analysis
Catheter Stabilization Device Catheter Securement Devices Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Catheter Stabilization Device Catheter Securement Devices Market Analysis Report by Region
Europe Catheter Stabilization Device Catheter Securement Devices Market Report:
The market in Europe is anticipated to grow from $0.54 billion in 2023 to $1.05 billion by 2033. The region benefits from stringent healthcare regulations fostering the adoption of advanced securement devices.Asia Pacific Catheter Stabilization Device Catheter Securement Devices Market Report:
In Asia Pacific, the market is expected to grow from $0.33 billion in 2023 to $0.64 billion by 2033, driven by rising healthcare expenditures and an increasing elderly population requiring catheter solutions.North America Catheter Stabilization Device Catheter Securement Devices Market Report:
North America holds a substantial market share, expected to grow from $0.69 billion in 2023 to $1.33 billion in 2033, emphasizing innovations in healthcare and a growing prevalence of chronic diseases.South America Catheter Stabilization Device Catheter Securement Devices Market Report:
South America’s market is projected to expand from $0.11 billion in 2023 to $0.22 billion by 2033 as healthcare infrastructure improves and awareness increases regarding the importance of catheter securement.Middle East & Africa Catheter Stabilization Device Catheter Securement Devices Market Report:
In the Middle East and Africa, the market is projected to reach $0.25 billion by 2033, up from $0.13 billion in 2023, motivated by improving healthcare systems and increased investments in medical technology.Tell us your focus area and get a customized research report.
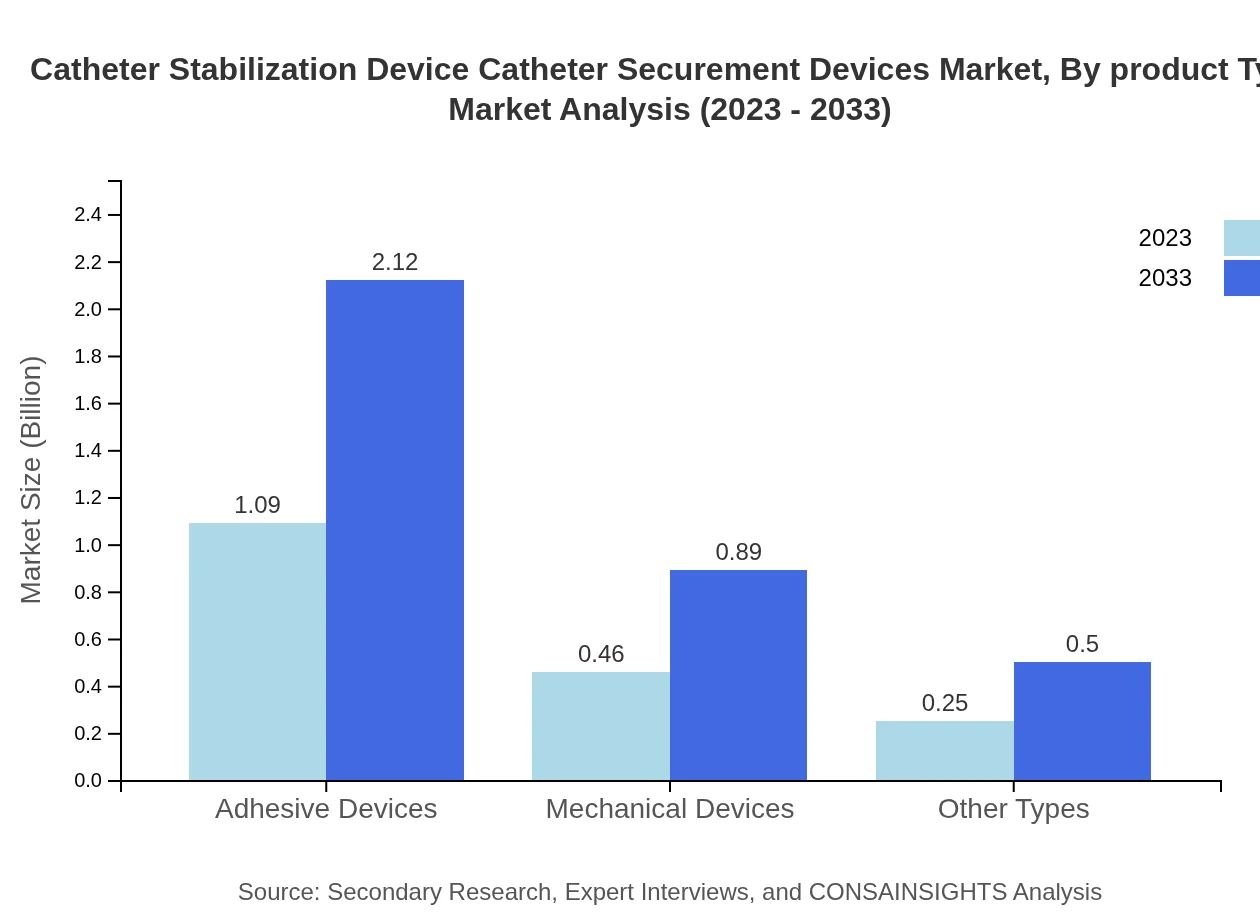
Catheter Stabilization Device Catheter Securement Devices Market Analysis By Product Type
The primary product types include adhesive devices and mechanical devices. Adhesive devices dominate the market, with expected growth from $1.09 billion in 2023 to $2.12 billion in 2033, attributed to their ease of use and effectiveness. Mechanical devices also show significant growth potential, doubling their market size in the same period.
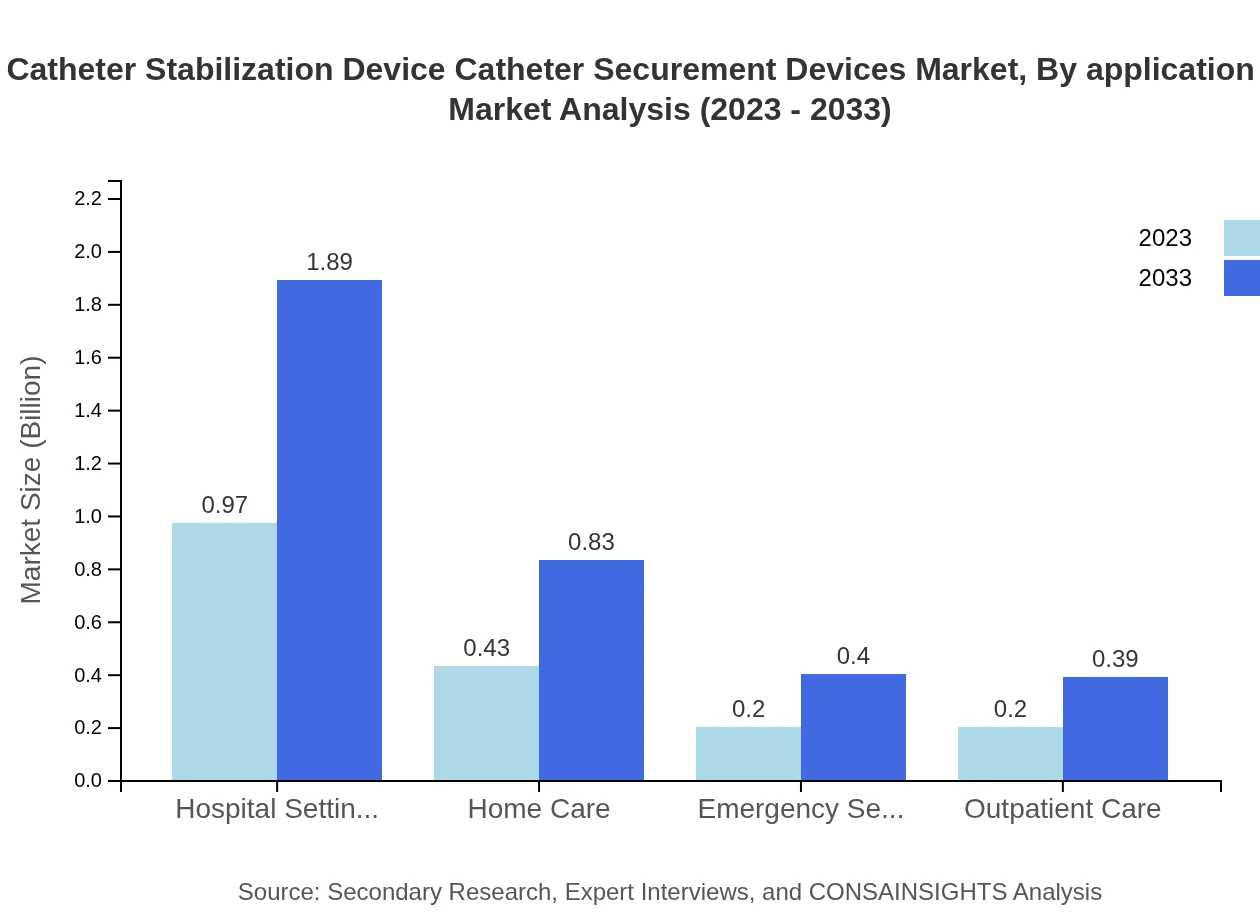
Catheter Stabilization Device Catheter Securement Devices Market Analysis By Application Area
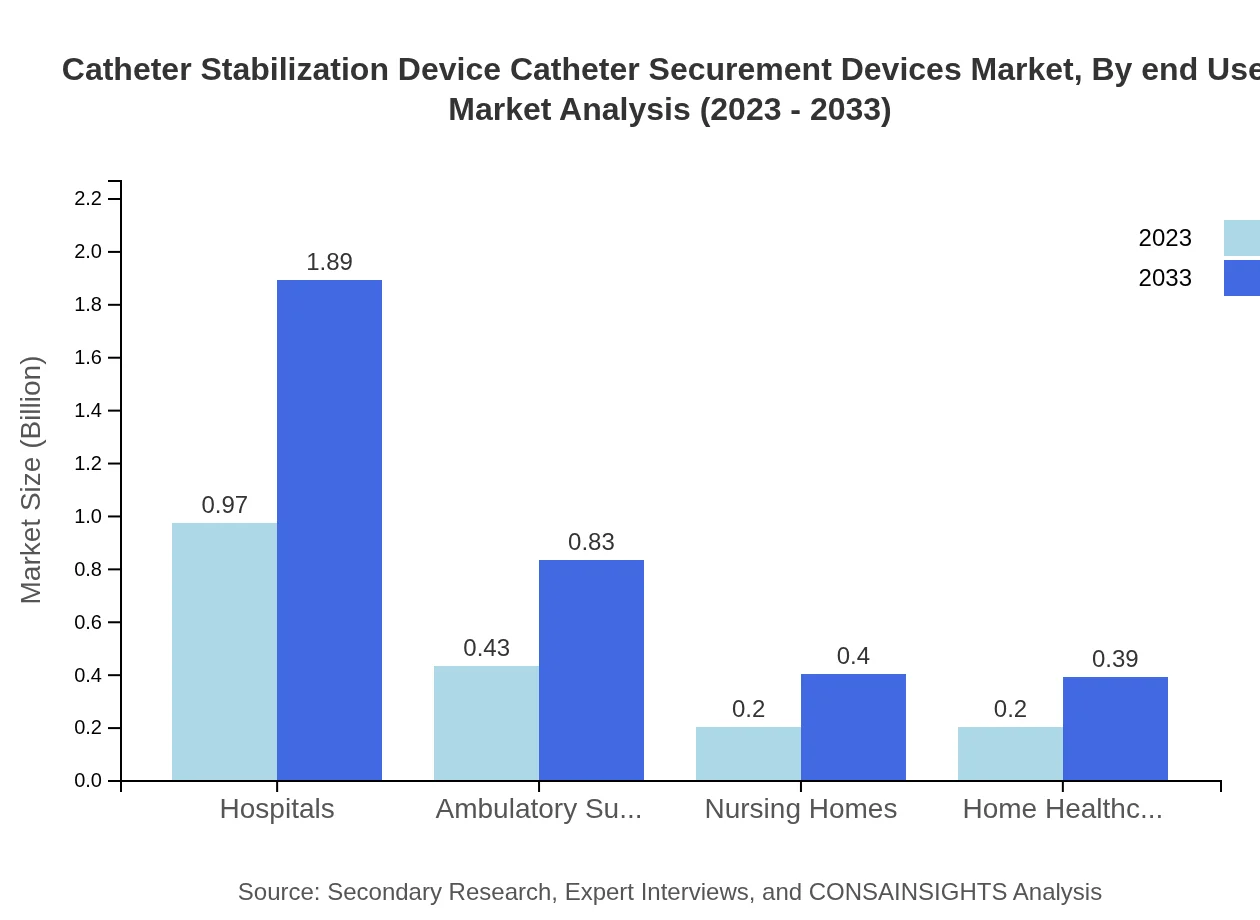
Key application areas consist of hospital settings and home care. Hospital settings lead the market, expected to grow from $0.97 billion in 2023 to $1.89 billion in 2033. Home care is also expanding, with a growth trajectory from $0.43 billion to $0.83 billion, reflecting a trend towards outpatient care.
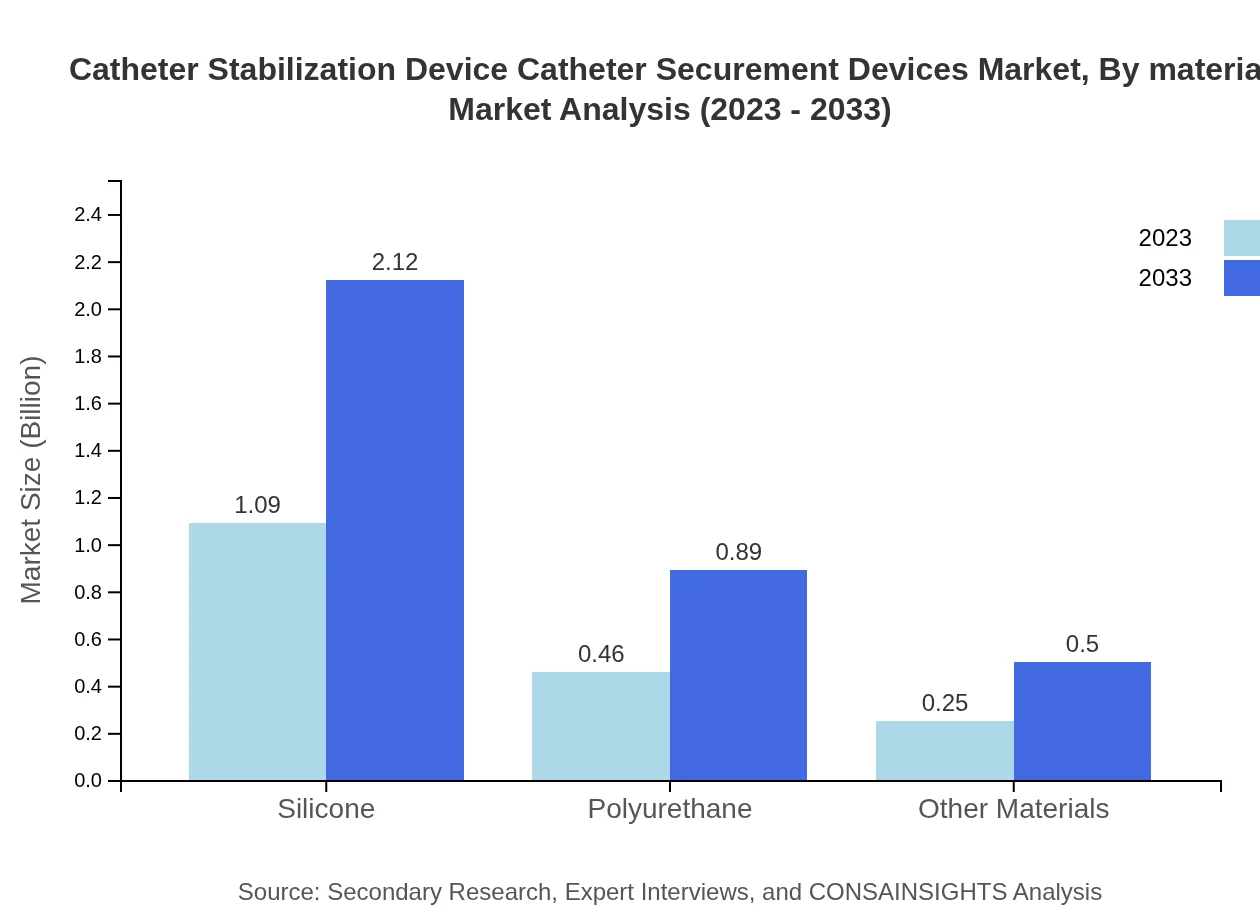
Catheter Stabilization Device Catheter Securement Devices Market Analysis By Material
Silicone is the leading material used in catheter stabilization devices, contributing significantly to the market at $1.09 billion in 2023, projected to grow to $2.12 billion by 2033. Polyurethane and other materials are also referenced but represent smaller portions of the market.
Catheter Stabilization Device Catheter Securement Devices Market Analysis By End User
End-users are segmented into hospitals, ambulatory surgery centers, nursing homes, and home healthcare. Hospitals account for the majority market share at 53.92% in 2023, projected to remain constant. Ambulatory surgery centers are also significant, showing a gradual increase in market share.
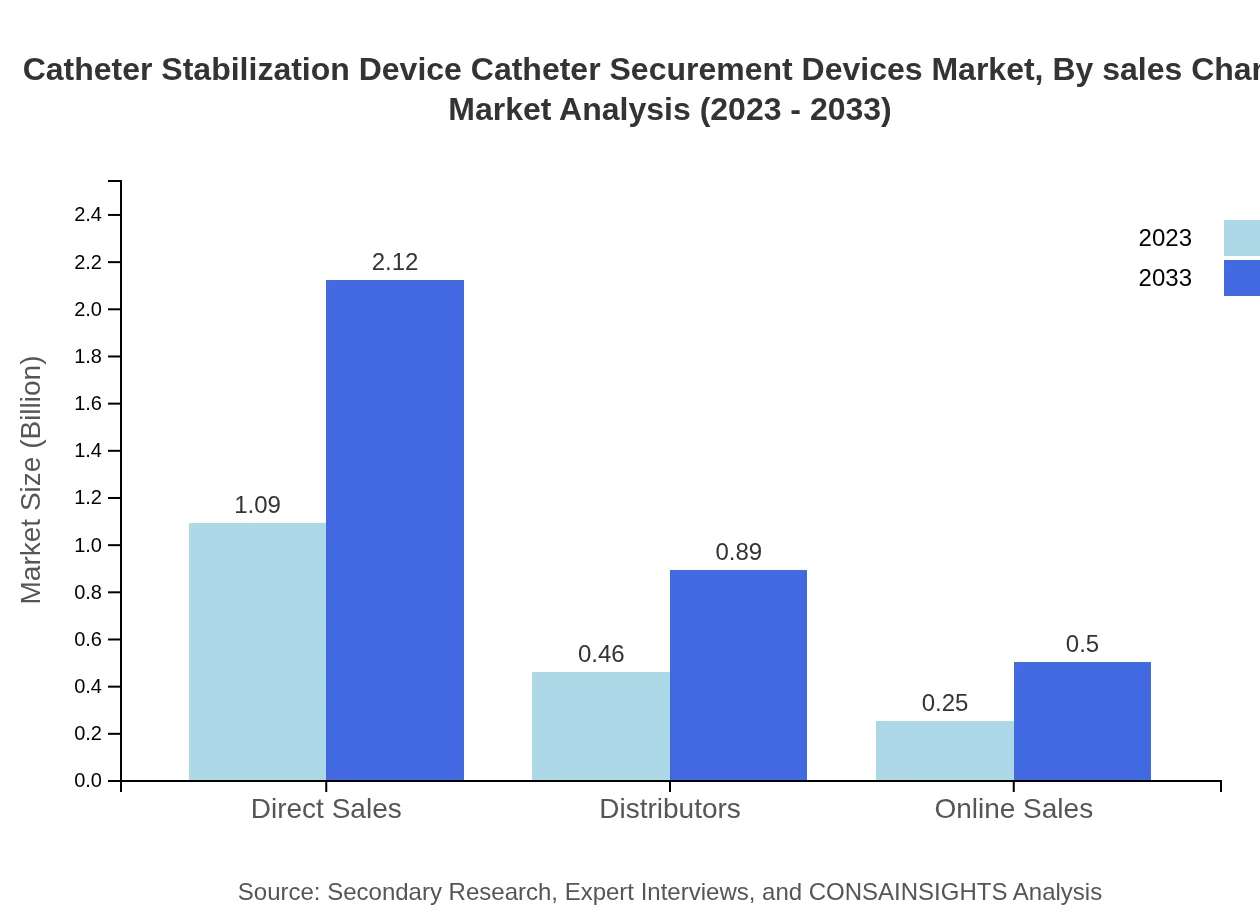
Catheter Stabilization Device Catheter Securement Devices Market Analysis By Sales Channel
Sales channels modeled include direct sales, distributors, and online sales. Direct sales currently dominate the market share at 60.52% in 2023; however, online sales are expected to gain traction over the forecast period, reflecting changing consumer purchasing behaviors.
Catheter Stabilization Device Catheter Securement Devices Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Catheter Stabilization Device Catheter Securement Devices Industry
Baxter International Inc.:
Baxter is a leading healthcare company dedicated to developing and marketing innovative catheters and securement devices, focusing on patient safety and advanced technology.3M Company:
3M is known for its rich portfolio in medical device technologies, including catheter securement solutions that enhance healing and minimize complications.Smith & Nephew:
Smith & Nephew is a global medical technology company that focuses on developing advanced wound management and catheter stabilization solutions.Medtronic Plc:
Medtronic is a leader in medical device innovation, significantly influencing the catheter stabilization device market with its pioneering securement solutions.Cardinal Health:
A prominent healthcare services and products company, Cardinal Health provides a range of catheter securement products aimed at improving patient care.We're grateful to work with incredible clients.









FAQs
What is the market size of catheter Stabilization Device Catheter Securement Devices?
The catheter stabilization device market is valued at $1.8 billion in 2023, with a projected CAGR of 6.7% over the next decade, indicating promising growth potential in this sector.
What are the key market players or companies in this catheter Stabilization Device Catheter Securement Devices industry?
Key players in this market include major healthcare companies focused on innovative catheter securement solutions. Their contributions are vital for advancements in patient safety and procedural efficacy.
What are the primary factors driving the growth in the catheter Stabilization Device Catheter Securement Devices industry?
Growth is driven by increasing procedures requiring catheters, advancements in securement technology, and heightened awareness regarding infection risks, prompting hospitals to adopt better practices.
Which region is the fastest Growing in the catheter Stabilization Device Catheter Securement Devices market?
The North American region exhibits significant growth, projected to rise from $0.69 billion in 2023 to $1.33 billion by 2033, driven by healthcare innovations and market demand.
Does ConsaInsights provide customized market report data for the catheter Stabilization Device Catheter Securement Devices industry?
Yes, ConsaInsights offers customized market report data tailored to specific needs in the catheter stabilization device sector, providing insights aligned with individual strategic goals.
What deliverables can I expect from this catheter Stabilization Device Catheter Securement Devices market research project?
Deliverables typically include comprehensive market reports, detailed analyses, segment breakdowns, and strategic recommendations, ensuring robust decision-making support for stakeholders.
What are the market trends of catheter Stabilization Device Catheter Securement Devices?
Market trends indicate increasing adoption of silicone-based securement devices, growth in home healthcare services, and a focus on minimizing infection risks, shaping future product development.