Cell Based Immunotherapy Market Report
Published Date: 31 January 2026 | Report Code: cell-based-immunotherapy
Cell Based Immunotherapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides comprehensive insights into the Cell Based Immunotherapy market, including market size projections, trends, and regional analyses from 2023 to 2033. The report aims to assist stakeholders in understanding current conditions and future opportunities within this growing sector.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
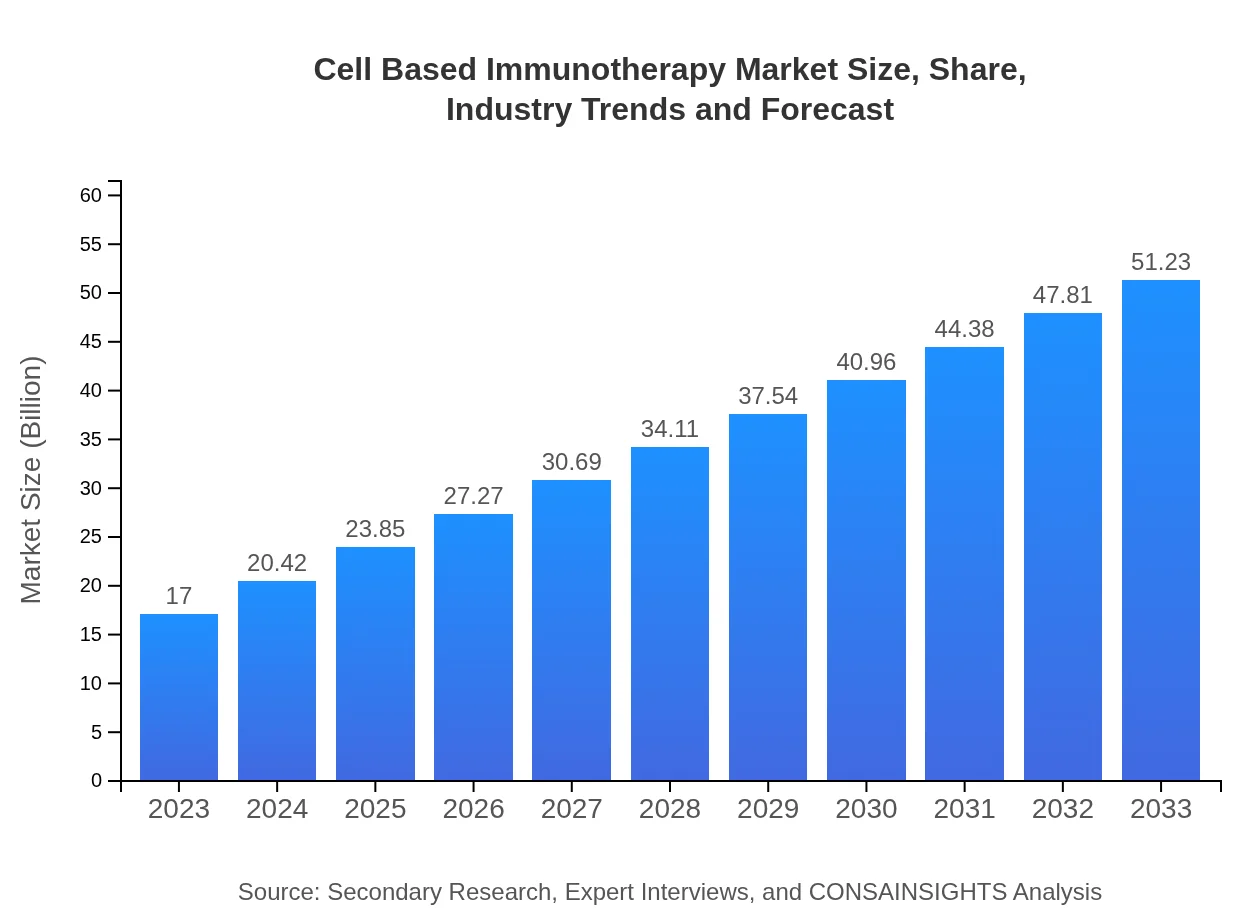
| 2023 Market Size | $17.00 Billion |
| CAGR (2023-2033) | 11.2% |
| 2033 Market Size | $51.23 Billion |
| Top Companies | Novartis, Gilead Sciences, Bristol-Myers Squibb, Amgen, Celyad Oncology |
| Last Modified Date | 31 January 2026 |
Cell Based Immunotherapy Market Overview
Customize Cell Based Immunotherapy Market Report market research report
- ✔ Get in-depth analysis of Cell Based Immunotherapy market size, growth, and forecasts.
- ✔ Understand Cell Based Immunotherapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cell Based Immunotherapy
What is the Market Size & CAGR of Cell Based Immunotherapy market in 2023?
Cell Based Immunotherapy Industry Analysis
Cell Based Immunotherapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cell Based Immunotherapy Market Analysis Report by Region
Europe Cell Based Immunotherapy Market Report:
The European market is expected to grow from $4.93 billion in 2023 to $14.85 billion by 2033. A supportive regulatory environment and increased funding for R&D in countries like Germany and the UK significantly boost the market's expansion.Asia Pacific Cell Based Immunotherapy Market Report:
In the Asia Pacific region, the market was valued at approximately $3.55 billion in 2023 and is projected to grow to $10.70 billion by 2033, showcasing a strong CAGR of around 12.3%. Factors like increasing investments in healthcare infrastructure, a surge in clinical trials, and innovative treatment methodologies contribute to this growth.North America Cell Based Immunotherapy Market Report:
North America remains the top market for Cell Based Immunotherapy, with a market size of $5.49 billion in 2023, anticipated to expand to $16.53 billion by 2033. The presence of major pharmaceutical companies, a robust clinical research framework, and high treatment adoption rates are pivotal factors behind this growth.South America Cell Based Immunotherapy Market Report:
The South American market, valued at $1.70 billion in 2023, is expected to reach $5.11 billion by 2033. The region is witnessing growing acceptance of advanced therapies driven by rising chronic diseases and strategic health care reforms aimed at enhancing the quality of care.Middle East & Africa Cell Based Immunotherapy Market Report:
In the Middle East and Africa, the market is projected to grow from $1.34 billion in 2023 to $4.04 billion by 2033, driven by a gradual increase in healthcare investments and awareness of cell-based therapies among healthcare providers.Tell us your focus area and get a customized research report.
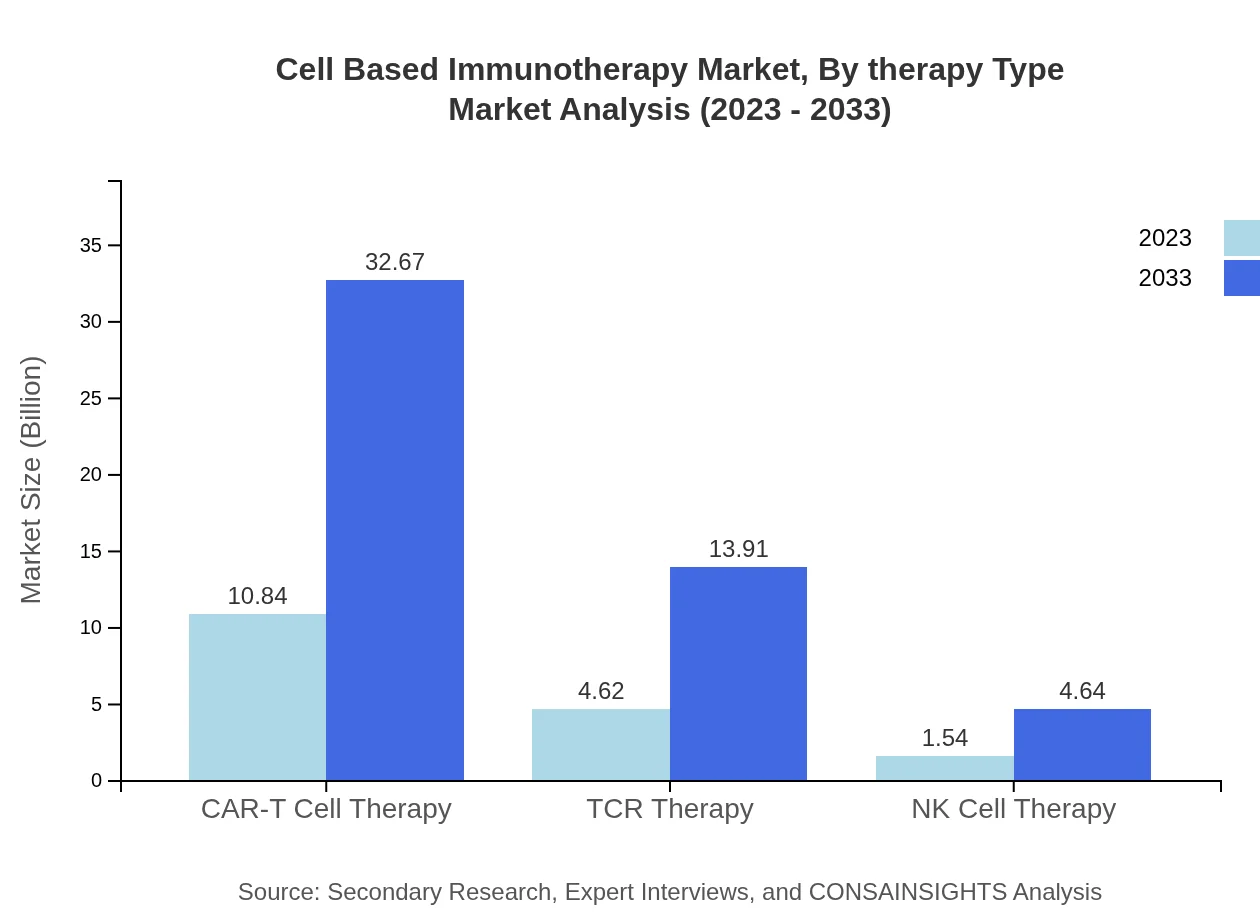
Cell Based Immunotherapy Market Analysis By Therapy Type
In 2023, the oncology segment alone accounted for approximately $10.84 billion, with a growth projection to $32.67 billion by 2033, reflecting its pivotal role in the market. CAR-T Cell Therapy leads this segment, endorsed by widespread clinical success and a robust pipeline of FDA approvals.
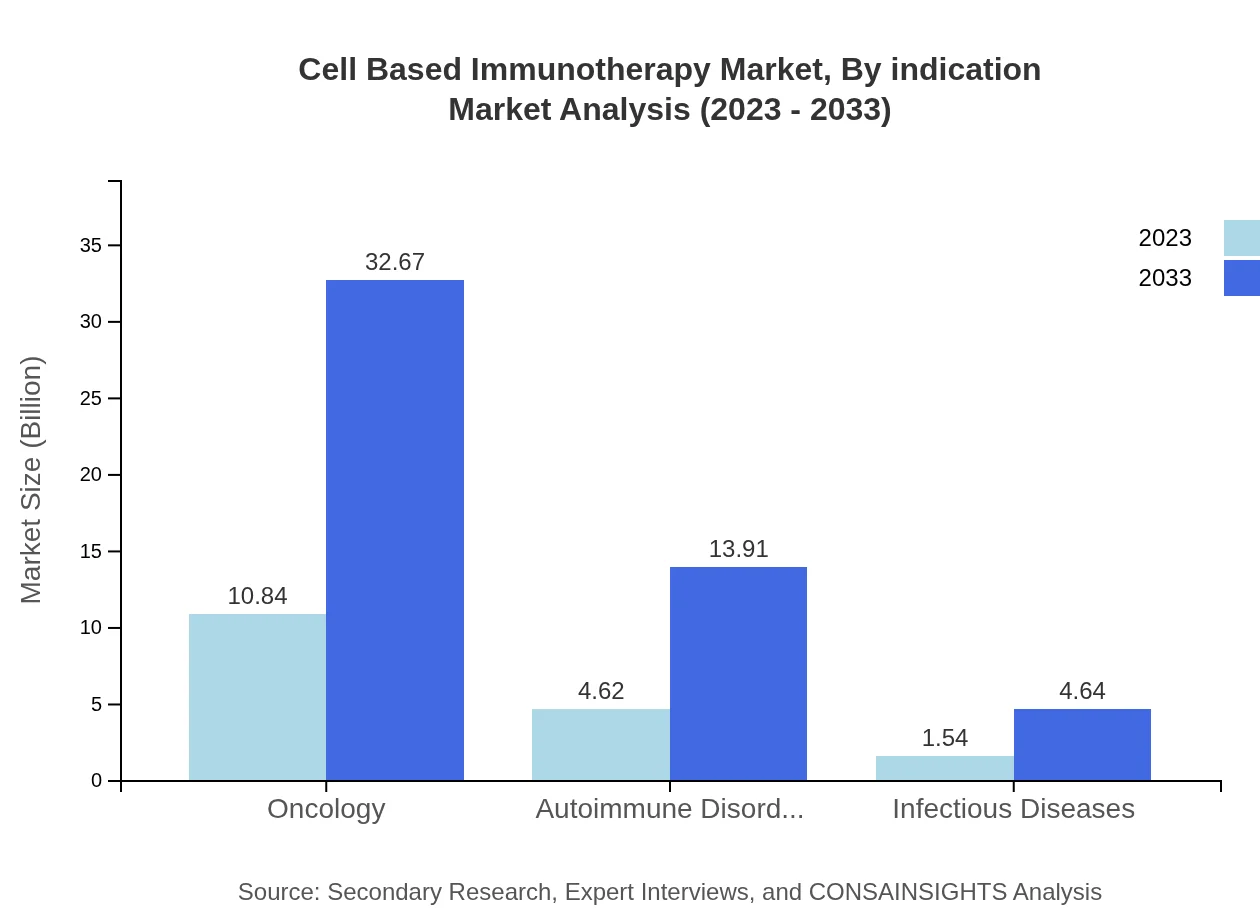
Cell Based Immunotherapy Market Analysis By Indication
Oncology accounts for 63.78% of the market share in 2023, indicating its dominance. Autoimmune disorders hold 27.16%, showcasing a significant portion of the market. The increasing prevalence of cancers globally enhances this segment's relevance.
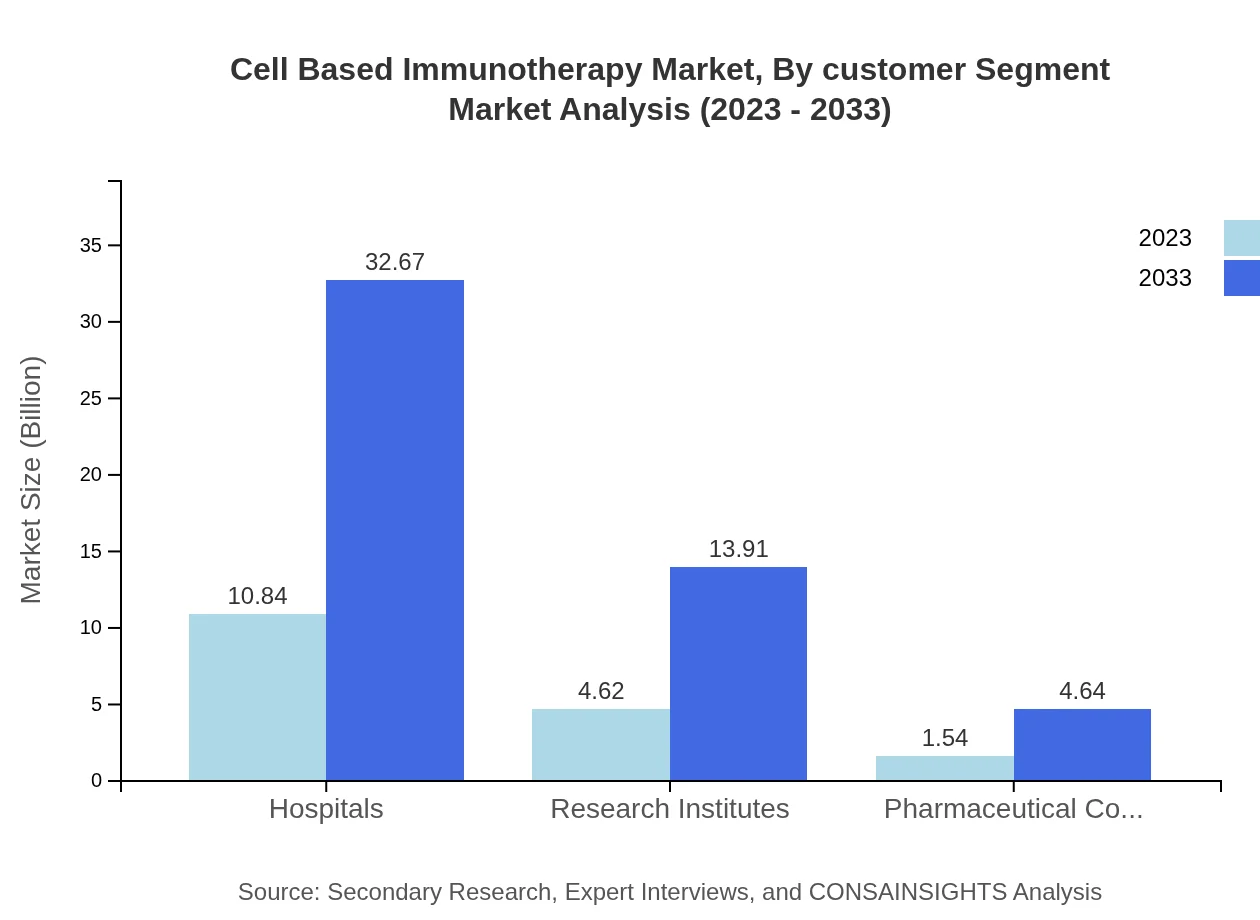
Cell Based Immunotherapy Market Analysis By Customer Segment
Hospitals represent the primary customer segment, contributing to 63.78% of the market in 2023. Research institutions and pharmaceutical companies also play substantial roles, accounting for 27.16% and 9.06% respectively, indicating diverse funding and application sources.
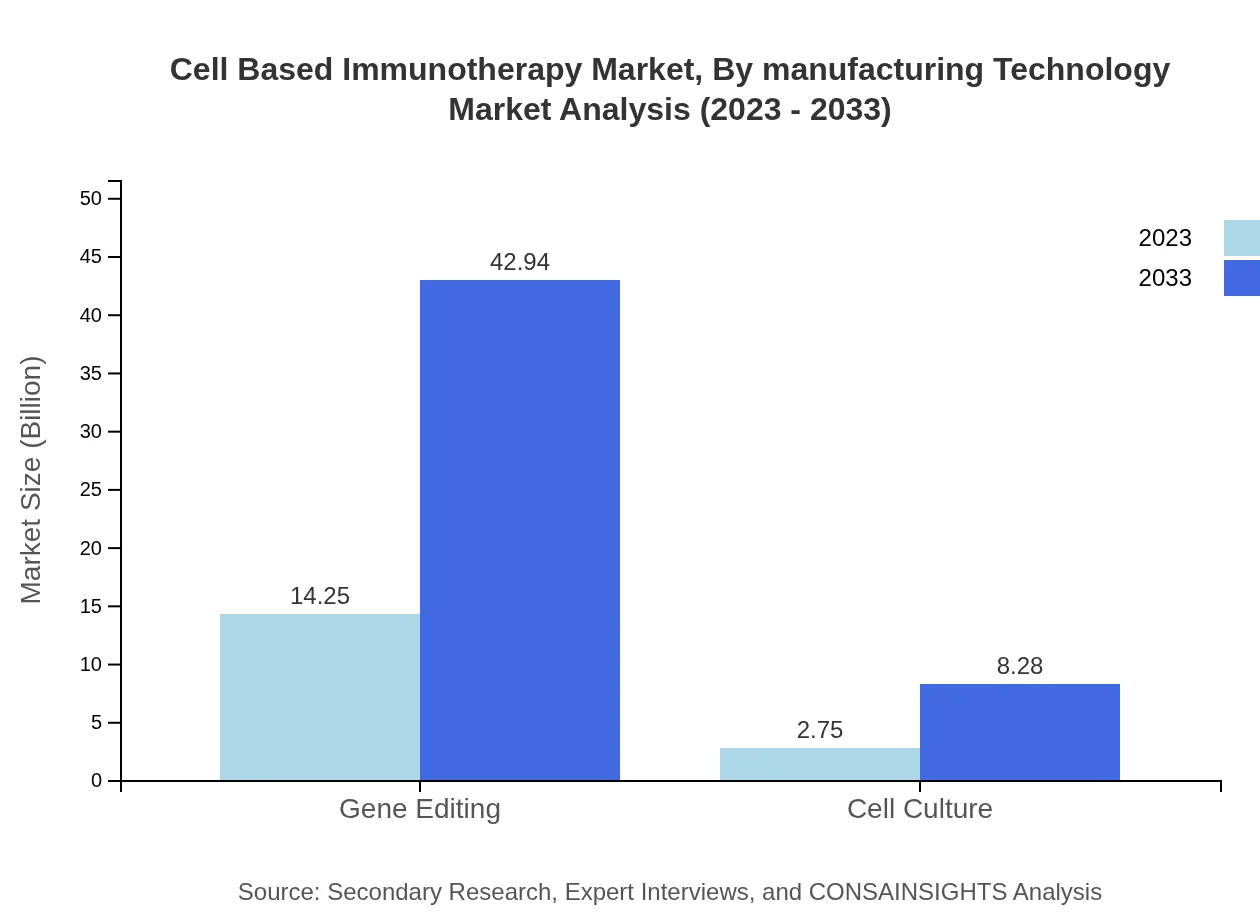
Cell Based Immunotherapy Market Analysis By Manufacturing Technology
Manufacturing technologies, including gene editing and cell culture, are projected to drive the market forward. Key developments in personalized medicine methodologies bolster this segment's growth, crucial for addressing diverse therapeutic needs.
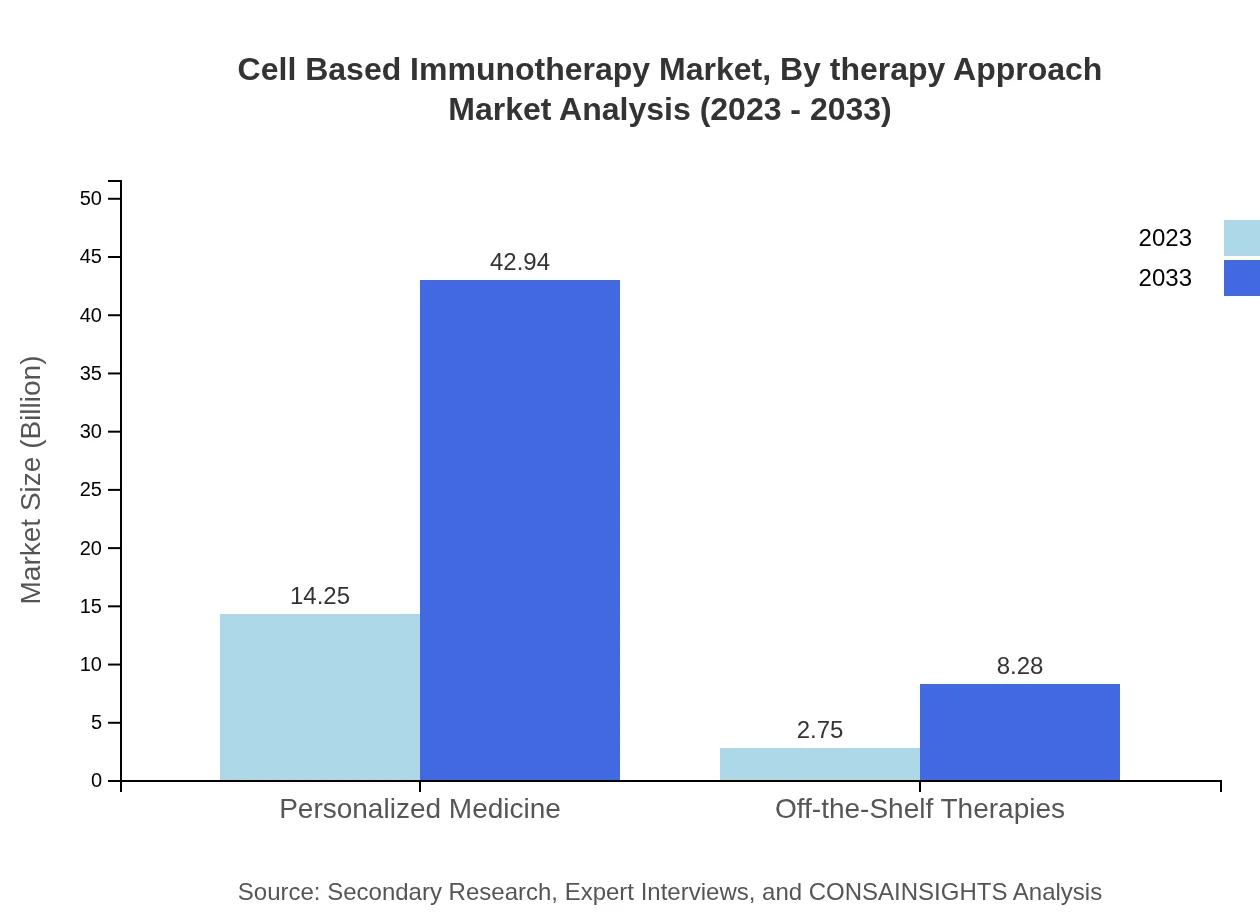
Cell Based Immunotherapy Market Analysis By Therapy Approach
Personalized medicine approaches dominate the therapy landscape with an 83.83% market share. Off-the-shelf therapies and gene editing technologies play supporting roles, facilitating rapid treatment solutions.
Cell Based Immunotherapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cell Based Immunotherapy Industry
Novartis:
A pioneer in CAR-T therapies, Novartis has developed Kymriah, showcasing significant impact in the oncology therapy market.Gilead Sciences:
Known for its innovative Yescarta, Gilead is a leader in providing cell therapies for various hematologic malignancies.Bristol-Myers Squibb:
BMS has expanded its offerings in CAR-T therapy with Breyanzi, advancing treatment options for lymphomas.Amgen:
Focusing on addressing unmet medical needs, Amgen invests in early-stage R&D for cell-based therapies.Celyad Oncology:
A biotech firm specializing in developing innovative therapies using engineered T-cells to target tumors.We're grateful to work with incredible clients.









FAQs
What is the market size of cell Based immunotherapy?
The cell-based immunotherapy market is projected to reach approximately $17 billion by 2033, with a CAGR of 11.2%. This growth is driven by increasing adoption and advancements in immunotherapy techniques.
What are the key market players or companies in the cell Based immunotherapy industry?
Key players in the cell-based immunotherapy market include major pharmaceutical companies and biotechs actively working on innovative therapies and treatments for cancer and autoimmune diseases. These companies focus on research and clinical advancements in CAR-T and TCR therapies.
What are the primary factors driving the growth in the cell Based immunotherapy industry?
Growth in the cell-based immunotherapy sector is primarily driven by increasing rates of cancer and autoimmune disorders, advancements in technology, and the rising demand for personalized medicine approaches that enhance treatment efficacy and safety.
Which region is the fastest Growing in the cell Based immunotherapy market?
The fastest-growing region in the cell-based immunotherapy market is Europe, projected to grow from $4.93 billion in 2023 to $14.85 billion by 2033. Asia Pacific is also expanding significantly, expected to rise from $3.55 billion to $10.70 billion.
Does ConsaInsights provide customized market report data for the cell Based immunotherapy industry?
Yes, ConsaInsights provides customized market report data tailored to specific needs in the cell-based immunotherapy industry, allowing clients to access detailed insights, market trends, and forecasts that suit their strategic goals.
What deliverables can I expect from this cell Based immunotherapy market research project?
Clients can expect comprehensive deliverables including market size analysis, growth forecasts, competitive landscape details, trend identification, and regional breakdowns, ensuring all essential insights are provided for informed decision-making.
What are the market trends of cell Based immunotherapy?
Current trends in the cell-based immunotherapy market include increasing investment in R&D, pursuit of CAR-T and TCR therapies, and a shift towards personalized medicine and off-the-shelf therapies aimed at improving patient accessibility and treatment effectiveness.