Cell Therapy Market Report
Published Date: 31 January 2026 | Report Code: cell-therapy
Cell Therapy Market Size, Share, Industry Trends and Forecast to 2033
This report provides an in-depth analysis of the Cell Therapy market, covering its current state, growth potential, market size forecasts for 2023-2033, and key trends driving innovation and investment in this field.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
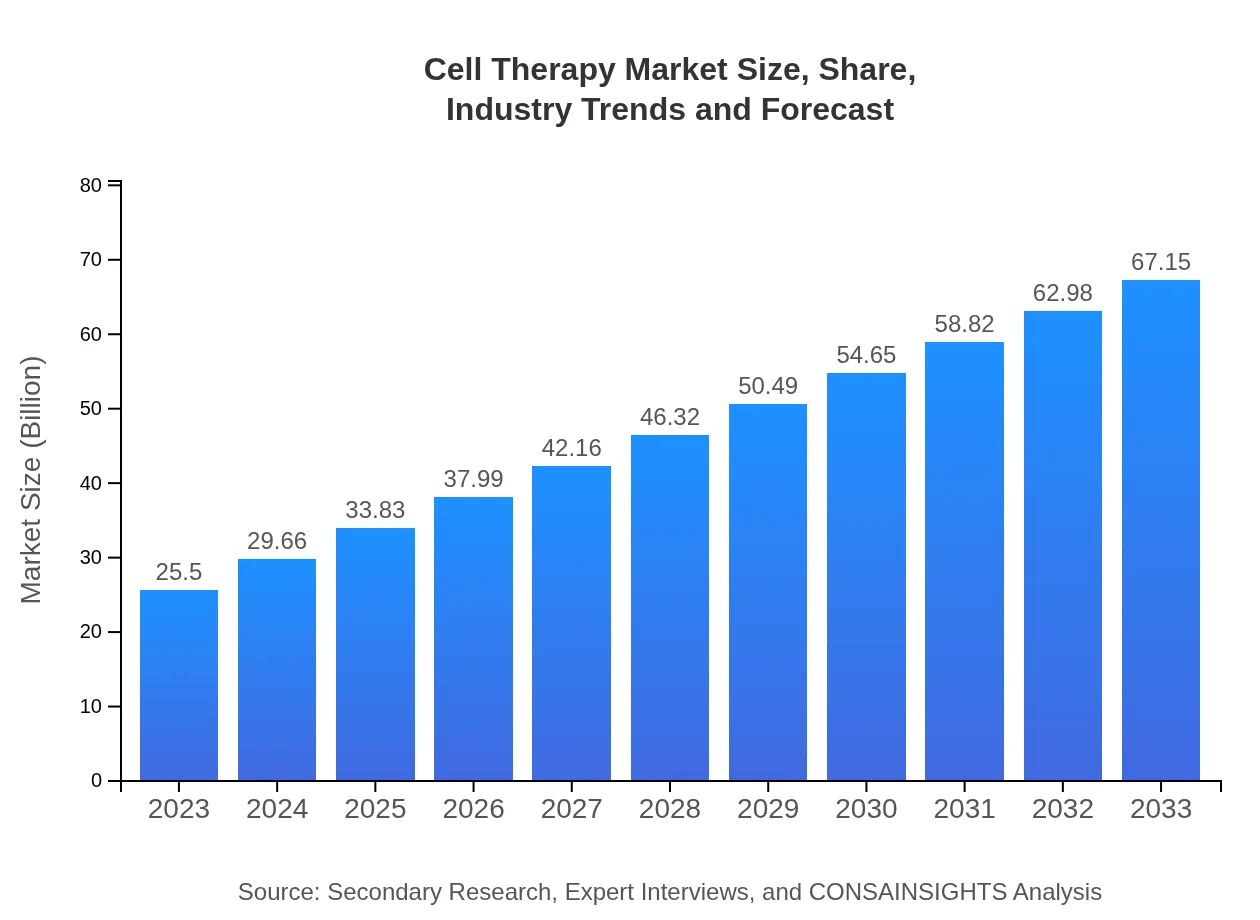
| 2023 Market Size | $25.50 Billion |
| CAGR (2023-2033) | 9.8% |
| 2033 Market Size | $67.15 Billion |
| Top Companies | Novartis, Gilead Sciences, Celgene Corporation (part of Bristol-Myers Squibb), AstraZeneca |
| Last Modified Date | 31 January 2026 |
Cell Therapy Market Overview
Customize Cell Therapy Market Report market research report
- ✔ Get in-depth analysis of Cell Therapy market size, growth, and forecasts.
- ✔ Understand Cell Therapy's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cell Therapy
What is the Market Size & CAGR of Cell Therapy market in 2023?
Cell Therapy Industry Analysis
Cell Therapy Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cell Therapy Market Analysis Report by Region
Europe Cell Therapy Market Report:
The European cell therapy market was valued at approximately USD 7.37 billion in 2023 and is forecasted to reach USD 19.41 billion by 2033. Extensive clinical research and an increasing number of cell therapy approvals in European countries are propelling the market.Asia Pacific Cell Therapy Market Report:
In 2023, the Asia Pacific cell therapy market is projected to be valued at USD 5.49 billion, growing to USD 14.45 billion by 2033. The region's growth is attributed to an increasing patient pool, rising healthcare expenditure, and strong government support for biotechnology and healthcare innovation.North America Cell Therapy Market Report:
North America holds the largest market share, expected to grow from USD 8.20 billion in 2023 to USD 21.59 billion by 2033. The robust healthcare infrastructure, high investment in R&D, and early adoption of cell-based therapies are significant growth drivers in this region.South America Cell Therapy Market Report:
The South America market is estimated to grow from USD 2.55 billion in 2023 to USD 6.71 billion by 2033. The demand for advanced treatment options and growing awareness regarding cell therapies drive this increase, despite economic challenges affecting healthcare expenditures.Middle East & Africa Cell Therapy Market Report:
The Middle East and Africa market is anticipated to grow from USD 1.89 billion in 2023 to USD 4.99 billion by 2033. The region faces healthcare accessibility challenges, but increasing investments in biotechnology and healthcare innovation provide significant growth opportunities.Tell us your focus area and get a customized research report.
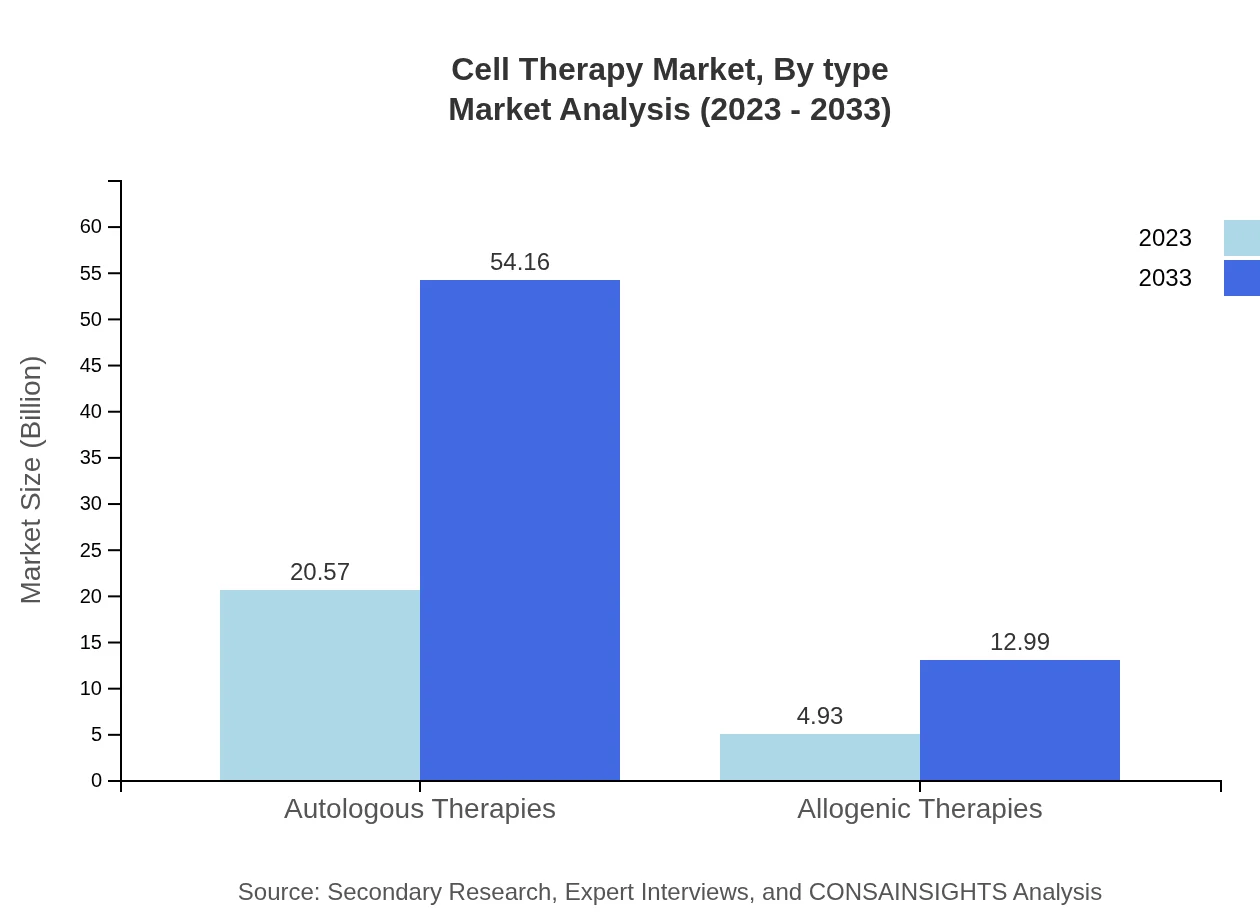
Cell Therapy Market Analysis By Type
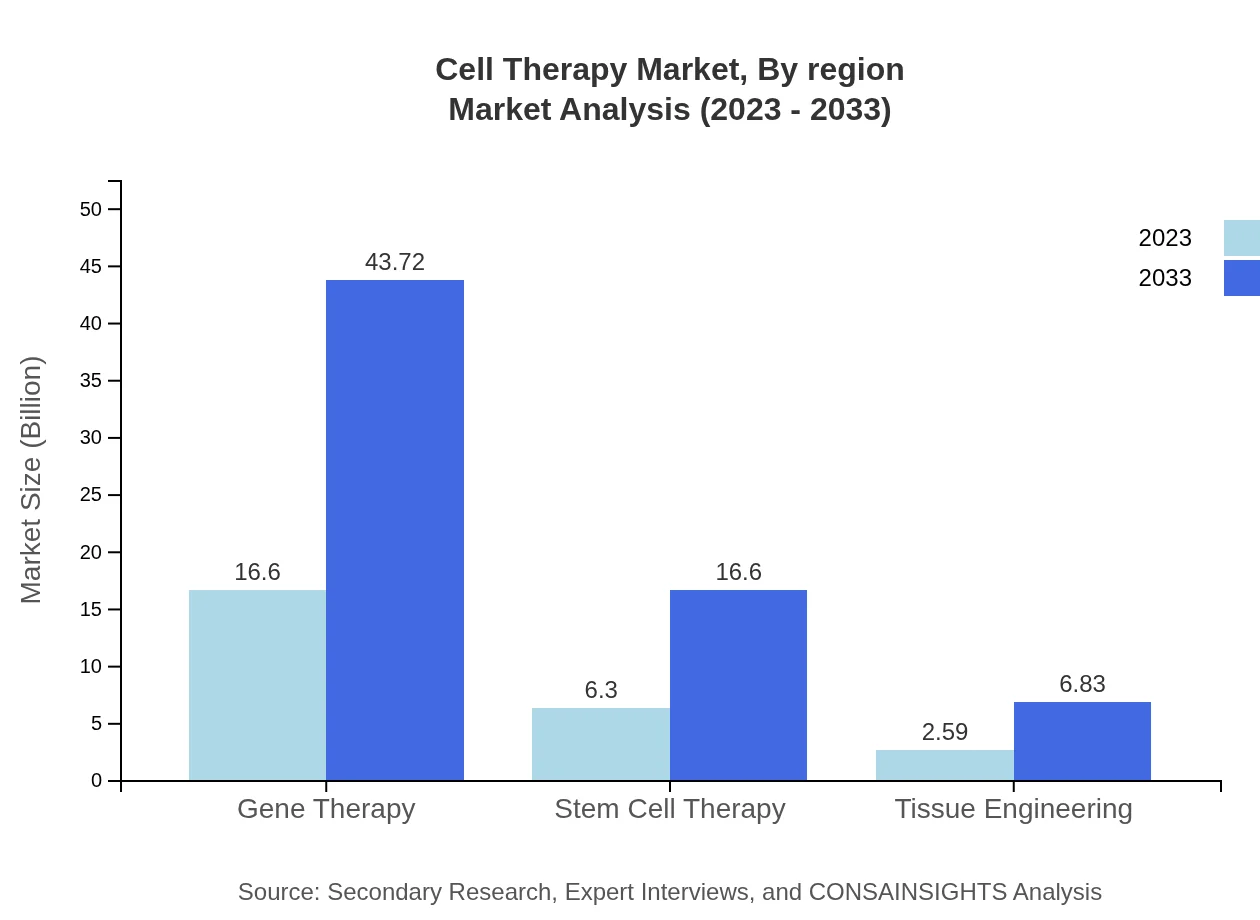
The market is primarily segmented into Autologous Therapies, Allogenic Therapies, Gene Therapy, Stem Cell Therapy, and Tissue Engineering. Autologous Therapies account for approximately USD 20.57 billion in 2023 and are projected to reach USD 54.16 billion by 2033. In contrast, Allogenic Therapies represent a smaller share, valued at USD 4.93 billion in 2023, increasing to USD 12.99 billion by 2033. Gene Therapy is also critical, projected to grow from USD 16.60 billion to USD 43.72 billion during the same period.
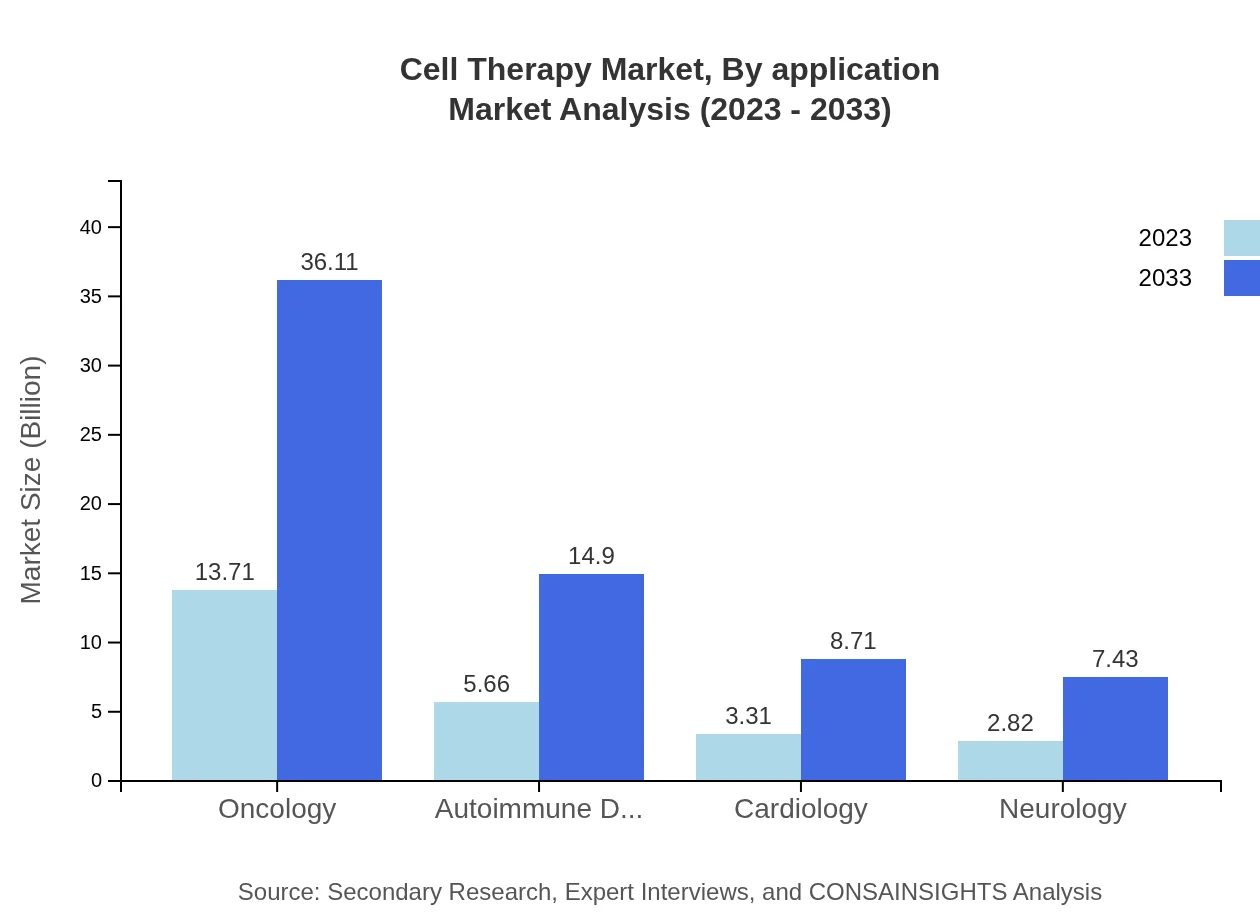
Cell Therapy Market Analysis By Application
The Cell Therapy market is segmented by application, including Oncology, Autoimmune disorders, Cardiology, and Neurology. Oncology holds a significant market share of approximately USD 13.71 billion in 2023 with forecasted growth to USD 36.11 billion by 2033, driven by increasing cancer incidences and treatment needs.
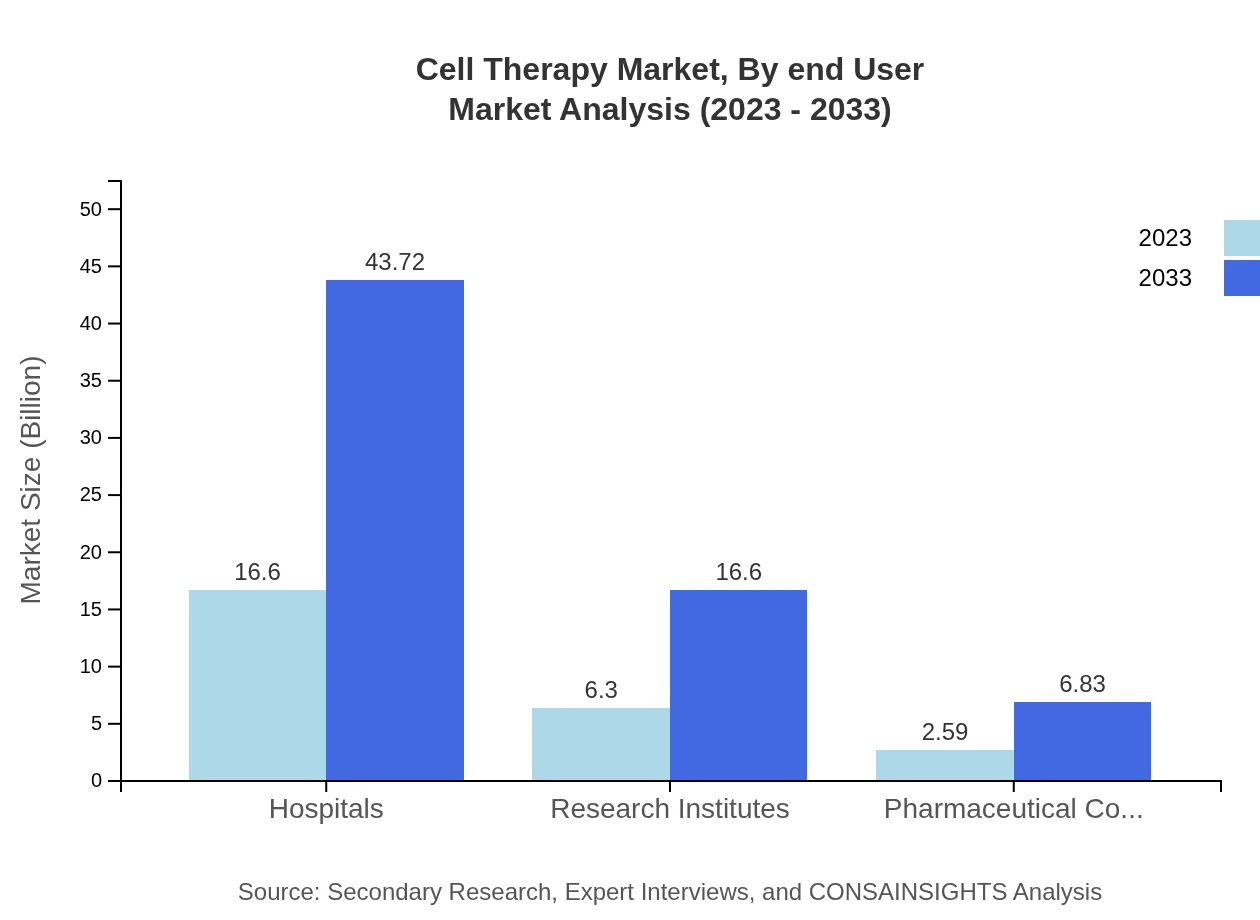
Cell Therapy Market Analysis By End User
The end-user segment includes Hospitals, Research Institutes, and Pharmaceutical Companies. Hospitals dominate the market, valued at USD 16.60 billion in 2023 and expected to reach USD 43.72 billion by 2033. Increasing patient load and technological advancements are enhancing hospital capacities for cell therapy treatments.
Cell Therapy Market Analysis By Region
The geographical breakdown shows North America leading the market, followed by Europe and Asia Pacific. Each region demonstrates unique growth dynamics driven by regulatory frameworks, healthcare infrastructure, and economic factors influencing cell therapy adoption.
Cell Therapy Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cell Therapy Industry
Novartis:
A global healthcare company focused on patented prescription medicines, particularly pioneering in CAR-T cell therapies, driving significant advancements in the treatment of blood cancers.Gilead Sciences:
Known for its innovative therapies, Gilead has made significant contributions to the Cell Therapy sector through its development of Yescarta, a key product in fighting large B-cell lymphoma.Celgene Corporation (part of Bristol-Myers Squibb):
Celgene has a strong presence in the Cell Therapy landscape with its cutting-edge CAR T-cell therapy, contributing to transformative treatments in hematology.AstraZeneca:
Focusing on innovation, AstraZeneca has invested heavily in cell therapy R&D, exploring potential treatments for various severe diseases.We're grateful to work with incredible clients.









FAQs
What is the market size of cell Therapy?
The global cell therapy market is valued at $25.5 billion in 2023, expected to grow at a CAGR of 9.8% from 2023 to 2033, indicating significant growth potential in this sector as it evolves.
What are the key market players or companies in the cell therapy industry?
Key players in the cell therapy industry include Novartis, Gilead, Amgen, and Celgene. These companies are at the forefront of innovation, developing therapies that address various chronic conditions and cancers.
What are the primary factors driving the growth in the cell therapy industry?
The growth of the cell therapy market is driven by factors such as advancements in technology, increasing incidence of chronic diseases, rising awareness of personalized medicine, and substantial investment in R&D, leading to innovative therapeutic options.
Which region is the fastest Growing in cell therapy?
North America is currently the fastest-growing region, projected to expand from $8.20 billion in 2023 to $21.59 billion by 2033, driven by robust healthcare infrastructure and significant investments in biotechnology.
Does ConsaInsights provide customized market report data for the cell therapy industry?
Yes, ConsaInsights offers customized market report data for the cell therapy industry, catering to specific client needs, allowing for tailored insights into market dynamics and competitive landscape.
What deliverables can I expect from this cell therapy market research project?
The cell therapy market research project will provide comprehensive reports, including market size estimates, growth projections, competitive analysis, segment breakdowns, and regional insights, ensuring a detailed understanding of the market.
What are the market trends of cell therapy?
Current trends in the cell therapy market include increased focus on autologous therapies, advancements in gene therapies, and a shift towards targeted treatments for oncology and autoimmune disorders, highlighting innovation and patient-centric approaches.