Cell Therapy Technologies Market Report
Published Date: 31 January 2026 | Report Code: cell-therapy-technologies
Cell Therapy Technologies Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cell Therapy Technologies market, including market size, growth forecasts, regional insights, and emerging trends between 2023 and 2033. It offers data and insights for stakeholders to make informed decisions about investments and strategies.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
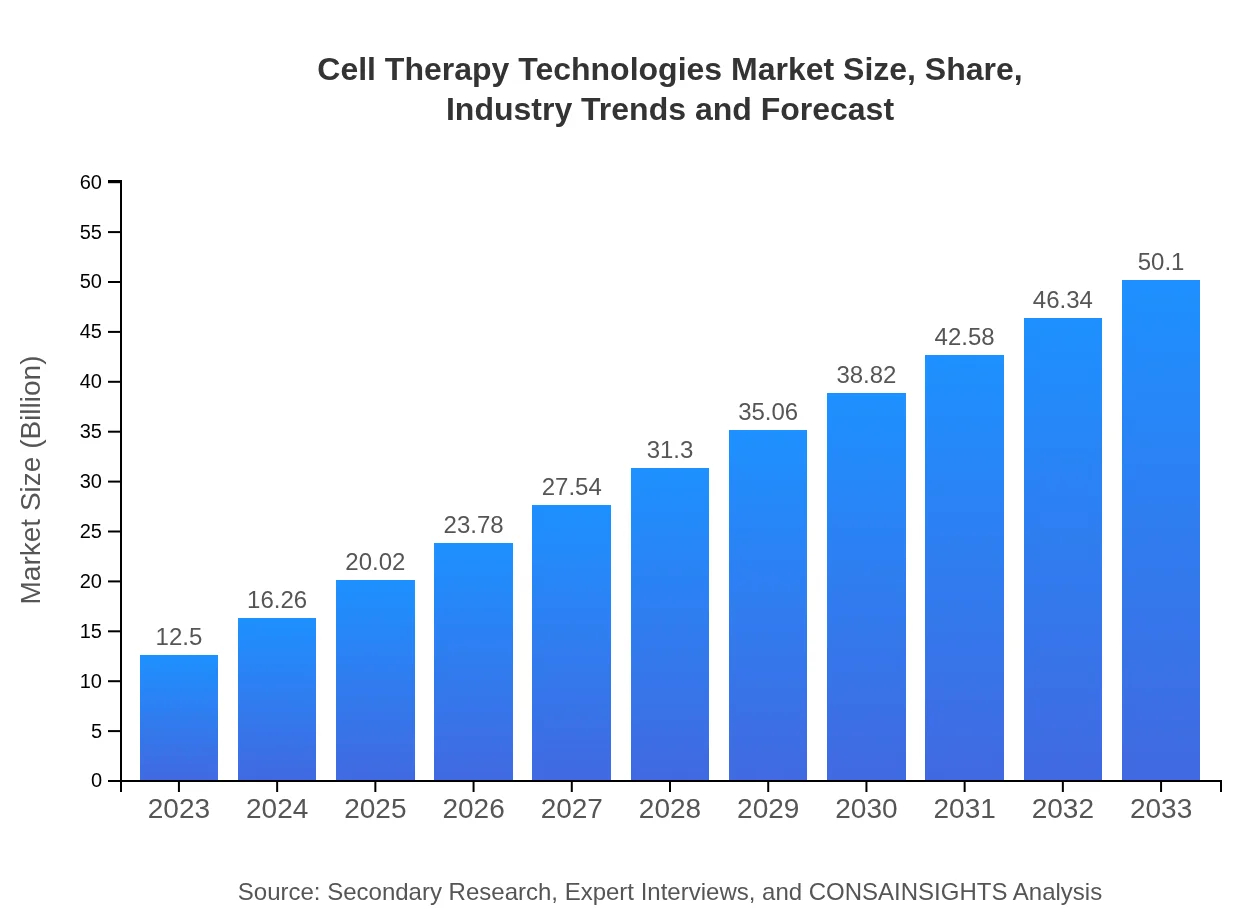
| 2023 Market Size | $12.50 Billion |
| CAGR (2023-2033) | 14.2% |
| 2033 Market Size | $50.10 Billion |
| Top Companies | Novartis, Gilead Sciences, Amgen, Bristol-Myers Squibb |
| Last Modified Date | 31 January 2026 |
Cell Therapy Technologies Market Overview
Customize Cell Therapy Technologies Market Report market research report
- ✔ Get in-depth analysis of Cell Therapy Technologies market size, growth, and forecasts.
- ✔ Understand Cell Therapy Technologies's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cell Therapy Technologies
What is the Market Size & CAGR of Cell Therapy Technologies market in 2023?
Cell Therapy Technologies Industry Analysis
Cell Therapy Technologies Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cell Therapy Technologies Market Analysis Report by Region
Europe Cell Therapy Technologies Market Report:
Europe's market is projected to experience substantial growth, escalating from $3.28 billion in 2023 to $13.13 billion by 2033. Increasing collaborations among key industry players and a robust research environment facilitate the accelerated advancement of cell therapy technologies.Asia Pacific Cell Therapy Technologies Market Report:
The Asia Pacific region is expected to experience substantial growth, transitioning from a market size of $2.68 billion in 2023 to $10.74 billion by 2033. Increased healthcare spending, developing medical infrastructure, and rising awareness of advanced therapies among patients are key drivers contributing to this growth.North America Cell Therapy Technologies Market Report:
North America remains the dominant market, poised to grow from $4.42 billion in 2023 to $17.72 billion by 2033. The heavy concentration of major pharmaceutical companies and research institutions, alongside strong regulatory support for innovative therapies, underscores the region's leadership in cell therapy development.South America Cell Therapy Technologies Market Report:
In South America, the market is growing steadily, with an anticipated increase from $0.67 billion in 2023 to $2.70 billion by 2033. The expansion of healthcare facilities and rising investment in biotechnology research are essential factors supporting this growth trajectory.Middle East & Africa Cell Therapy Technologies Market Report:
The Middle East and Africa market is expected to rise from $1.45 billion in 2023 to $5.81 billion by 2033. The growth can be attributed to increasing healthcare investments and expanding healthcare access, complemented by the establishment of regional research hubs.Tell us your focus area and get a customized research report.
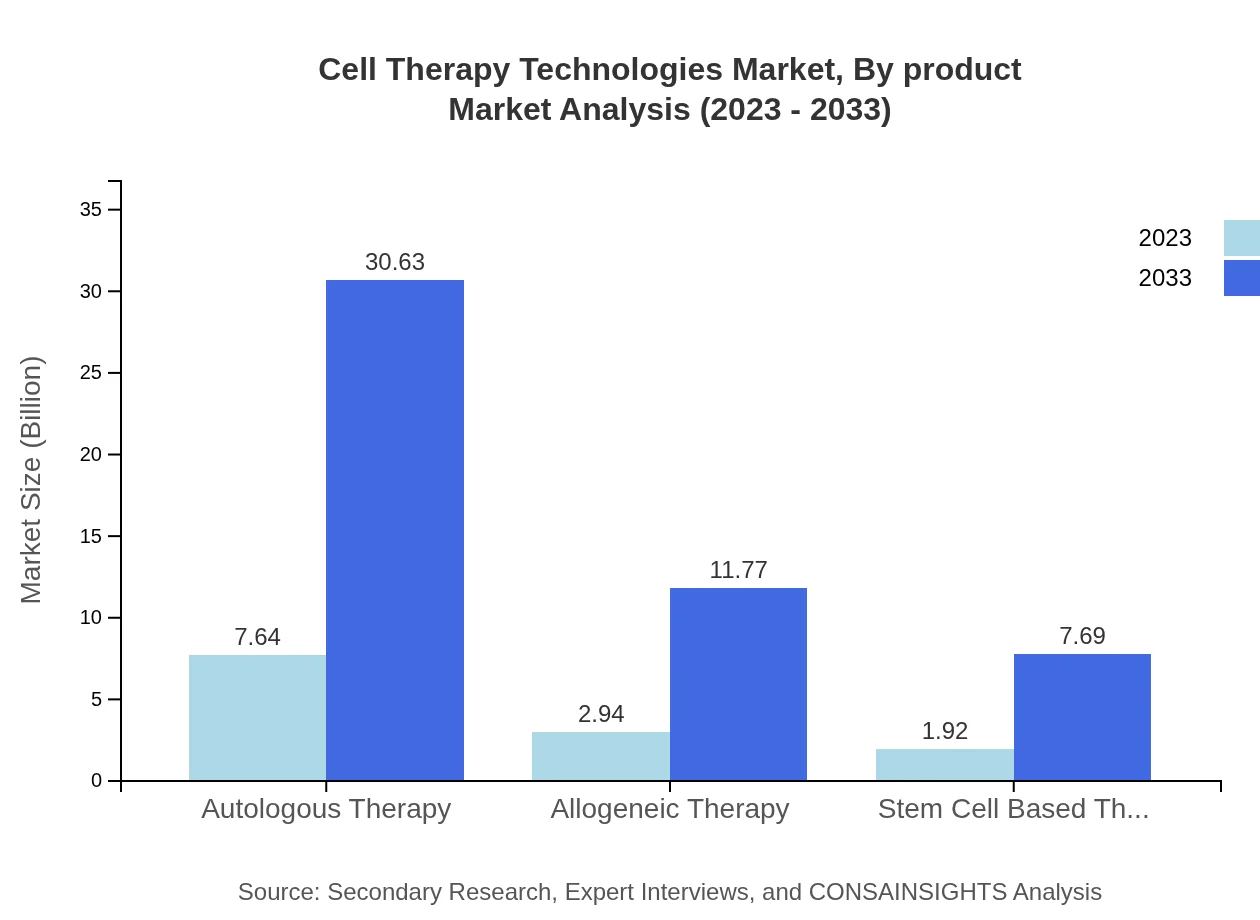
Cell Therapy Technologies Market Analysis By Product
The product segmentation highlights the scalability of both autologous and allogeneic therapies. By 2033, the market size for autologous therapies is projected to rise from $7.64 billion in 2023 to $30.63 billion, whereas allogeneic therapies are expected to grow from $2.94 billion to $11.77 billion, showcasing a strong demand for more versatile treatment options.
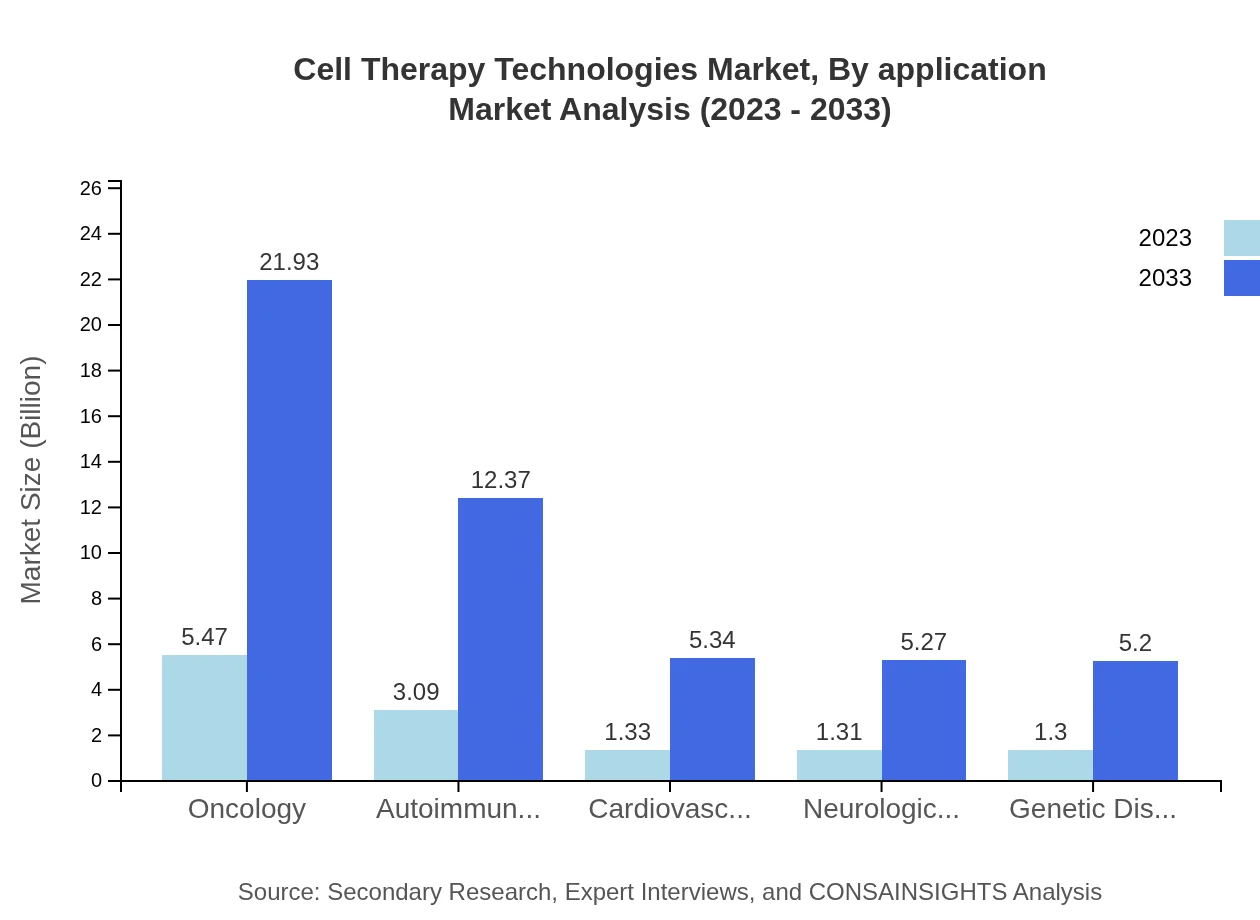
Cell Therapy Technologies Market Analysis By Application
In terms of application, the oncology segment leads the market with a 43.77% share in 2023, translating to $5.47 billion, anticipated to reach $21.93 billion by 2033. Other significant segments include autoimmune disorders and neurological disorders, indicating a diverse application horizon for cell therapies.
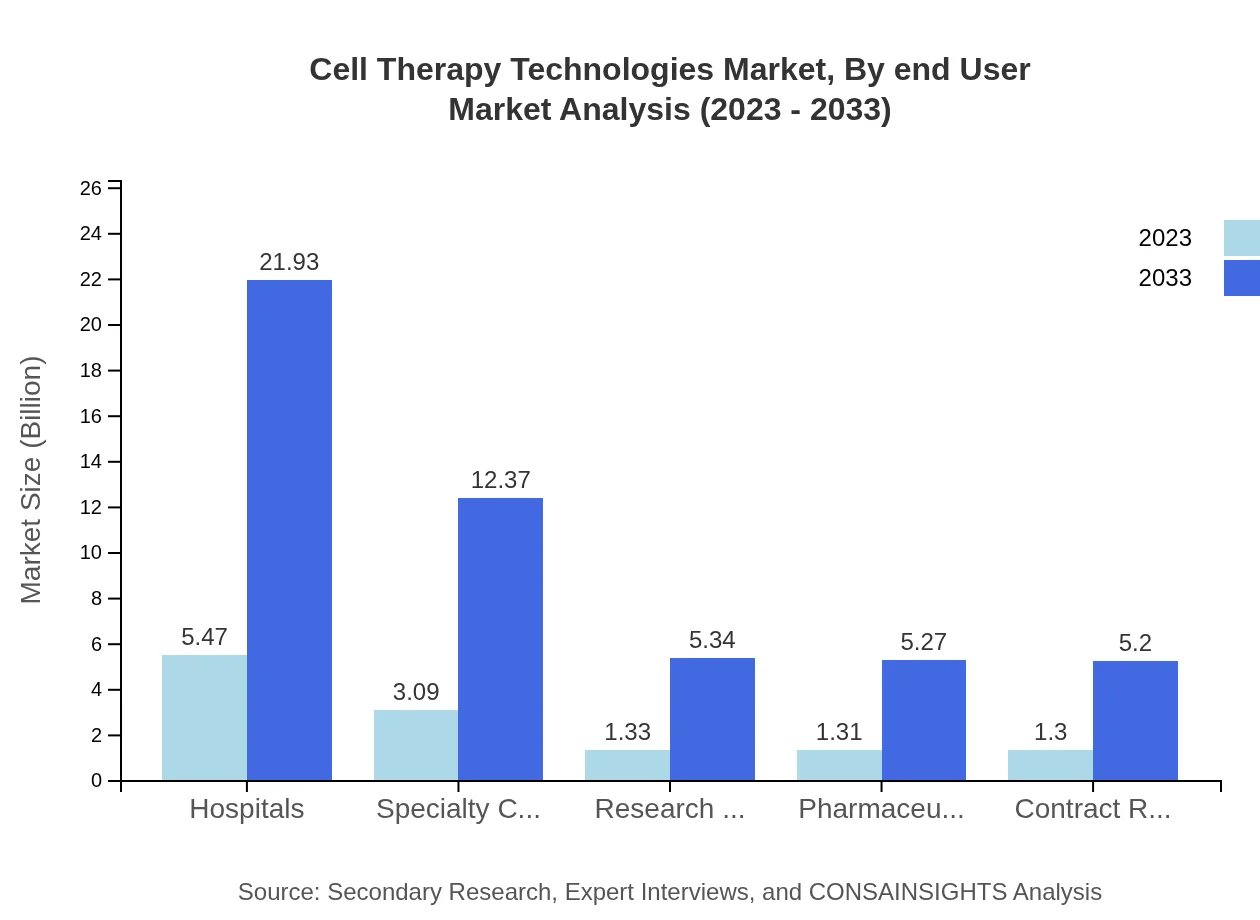
Cell Therapy Technologies Market Analysis By End User
The end-user analysis reveals hospitals' significant market share, at 43.77% in 2023, worth $5.47 billion, projected to expand to $21.93 billion by 2033. Specialty clinics and research organizations also play critical roles in delivering cell therapies to patients.
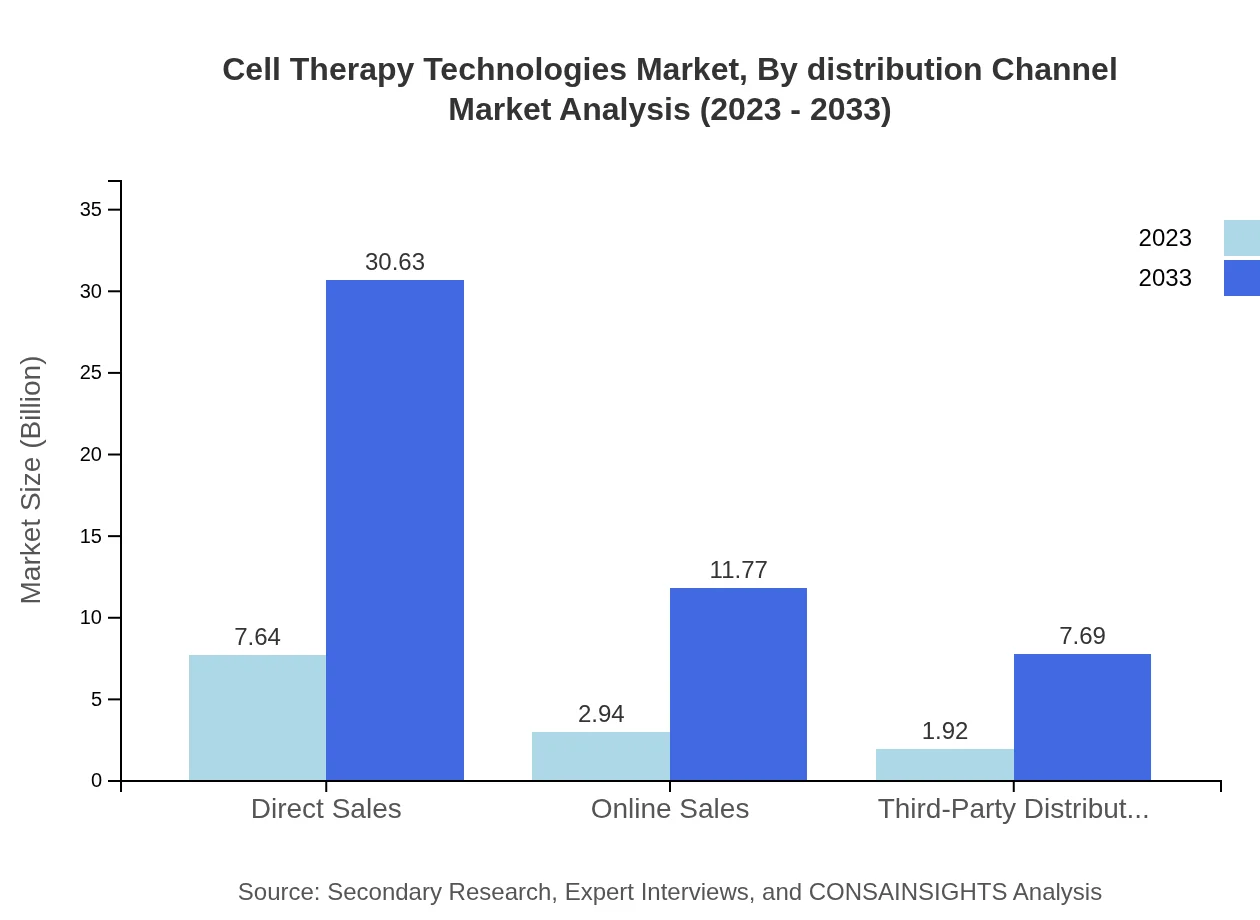
Cell Therapy Technologies Market Analysis By Distribution Channel
Direct sales dominate the distribution channels, holding a major share at 61.14% in 2023 and expected to rise to $30.63 billion by 2033. Online sales and third-party distributors are also growing, particularly as digital transformation reshapes healthcare delivery mechanisms.
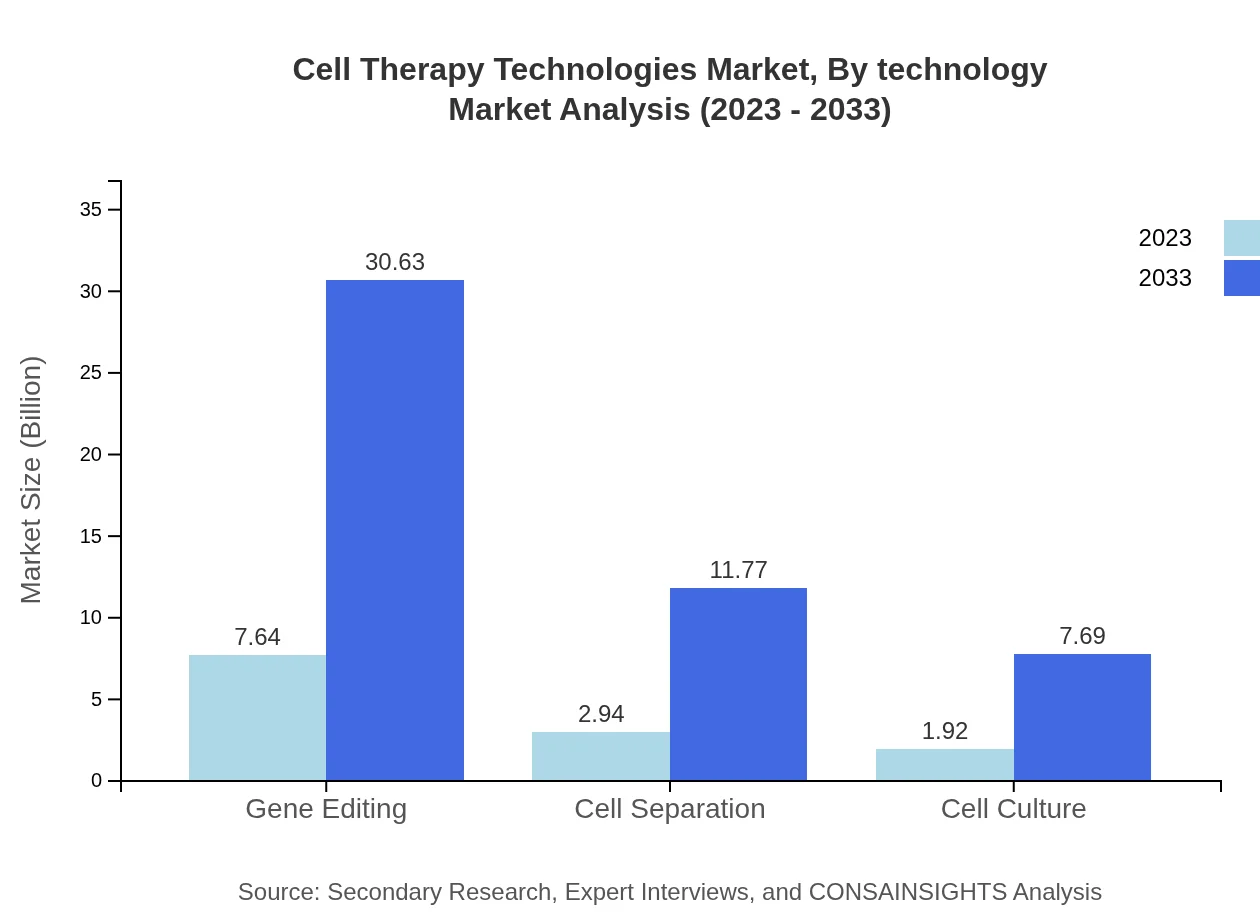
Cell Therapy Technologies Market Analysis By Technology
Key technologies influencing the market include gene editing, cell separation, and cell culture techniques. Gene editing technologies are projected to maintain a leading position, expected to expand from $7.64 billion in 2023 to $30.63 billion by 2033, driven by advancements and rising interest in personalized medicine.
Cell Therapy Technologies Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cell Therapy Technologies Industry
Novartis:
A pioneering leader in the cell therapy space known for developing Kymriah, the first CAR-T cell therapy for treating certain types of blood cancers.Gilead Sciences:
A key player specializing in advanced therapies, particularly in CAR-T and other cellular treatment modalities, focusing on hematology applications.Amgen:
Widely recognized for its innovative therapies and research in cell-based products, Amgen plays a substantial role in the biotechnology sector surrounding cellular applications.Bristol-Myers Squibb:
Known for its commitment to advancing immuno-oncology, the company has invested substantially in cell therapy products aimed at treating complicated blood cancers.We're grateful to work with incredible clients.









FAQs
What is the market size of cell Therapy Technologies?
The cell therapy technologies market is projected to grow from USD 12.5 billion in 2023 to undefined by 2033, at a CAGR of 14.2%. This growth indicates a significant increase in demand for advanced treatment options.
What are the key market players or companies in this cell Therapy Technologies industry?
Key players in the cell therapy technologies market include major pharmaceutical companies and biotech firms. Leading organizations are actively engaged in research and development, focusing on therapies like autologous and allogeneic treatments to push the industry forward.
What are the primary factors driving the growth in the cell therapy technologies industry?
The growth of the cell therapy technologies market is driven by several factors, including technological advancements, increased investment in research and development, rising incidences of chronic diseases, and a growing demand for personalized medicine.
Which region is the fastest Growing in the cell therapy technologies?
The fastest-growing region in the cell therapy technologies market is North America, projected to grow from USD 4.42 billion in 2023 to USD 17.72 billion by 2033. Europe and Asia Pacific are also experiencing significant growth.
Does ConsaInsights provide customized market report data for the cell therapy technologies industry?
Yes, ConsaInsights provides customized market report data for the cell therapy technologies industry. Clients can request specific data tailored to their needs, including detailed market analysis and forecasts.
What deliverables can I expect from this cell Therapy Technologies market research project?
From this market research project, you can expect comprehensive reports detailing market size, growth projections, competitive landscape analysis, and insights into regional and segment data tailored to the cell therapy technologies market.
What are the market trends of cell Therapy Technologies?
Current market trends in cell therapy technologies include an increase in the adoption of gene editing techniques, growth in stem cell therapies, and a shift towards more personalized treatment options, indicating a dynamic sector poised for innovation.