Cephalosporin Drugs Market Report
Published Date: 31 January 2026 | Report Code: cephalosporin-drugs
Cephalosporin Drugs Market Size, Share, Industry Trends and Forecast to 2033
This report provides a comprehensive analysis of the Cephalosporin Drugs market, offering insights into market trends, segmentation, regional analysis, and forecasts from 2023 to 2033.
| Metric | Value |
|---|---|
| Study Period | 2023 - 2033 |
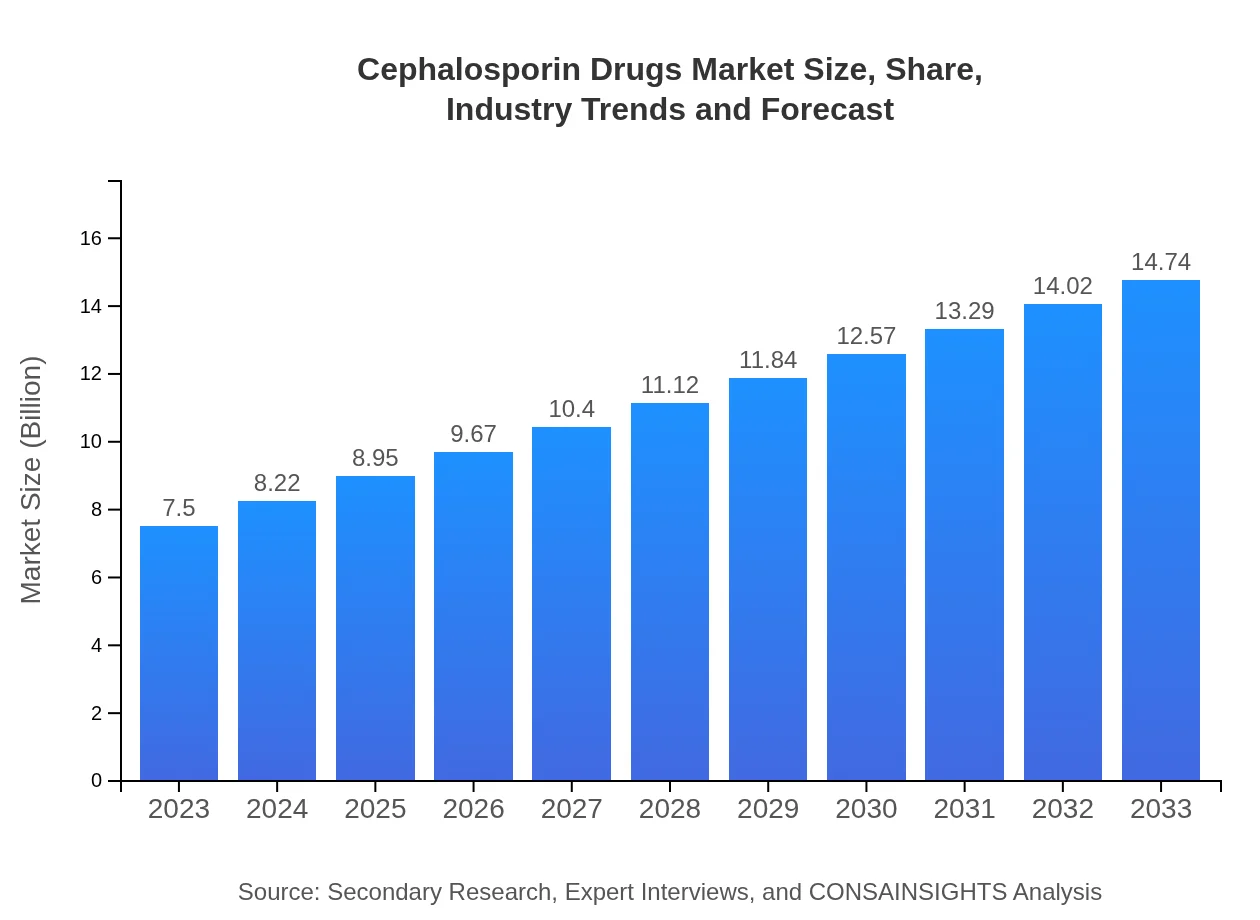
| 2023 Market Size | $7.50 Billion |
| CAGR (2023-2033) | 6.8% |
| 2033 Market Size | $14.74 Billion |
| Top Companies | Pfizer Inc., Merck & Co., Novartis AG, Johnson & Johnson, Roche Holdings AG |
| Last Modified Date | 31 January 2026 |
Cephalosporin Drugs Market Overview
Customize Cephalosporin Drugs Market Report market research report
- ✔ Get in-depth analysis of Cephalosporin Drugs market size, growth, and forecasts.
- ✔ Understand Cephalosporin Drugs's regional dynamics and industry-specific trends.
- ✔ Identify potential applications, end-user demand, and growth segments in Cephalosporin Drugs
What is the Market Size & CAGR of Cephalosporin Drugs market in 2023?
Cephalosporin Drugs Industry Analysis
Cephalosporin Drugs Market Segmentation and Scope
Tell us your focus area and get a customized research report.
Cephalosporin Drugs Market Analysis Report by Region
Europe Cephalosporin Drugs Market Report:
Europe's market is anticipated to grow from $2.18 billion in 2023 to $4.29 billion by 2033. This growth can be connected to supportive regulatory frameworks and rising healthcare needs across various European countries.Asia Pacific Cephalosporin Drugs Market Report:
In the Asia Pacific region, the cephalosporin drugs market is expected to grow from $1.49 billion in 2023 to $2.93 billion by 2033. This growth is attributed to increasing incidences of infectious diseases and higher healthcare expenditures in countries such as China and India.North America Cephalosporin Drugs Market Report:
North America holds a significant share of the market, with sizes expanding from $2.64 billion in 2023 to approximately $5.18 billion in 2033. The dominance of this region is fueled by strong research capabilities, high healthcare spending, and increasing antibiotic prescriptions.South America Cephalosporin Drugs Market Report:
The South American market will see growth from $0.69 billion in 2023 to $1.35 billion in 2033. Growth drivers include the rising prevalence of infectious diseases and improvements in healthcare infrastructure.Middle East & Africa Cephalosporin Drugs Market Report:
The Middle East and Africa market is projected to increase from $0.51 billion in 2023 to $0.99 billion in 2033, reflecting gradual improvements in healthcare access and a growing focus on combating infectious diseases.Tell us your focus area and get a customized research report.
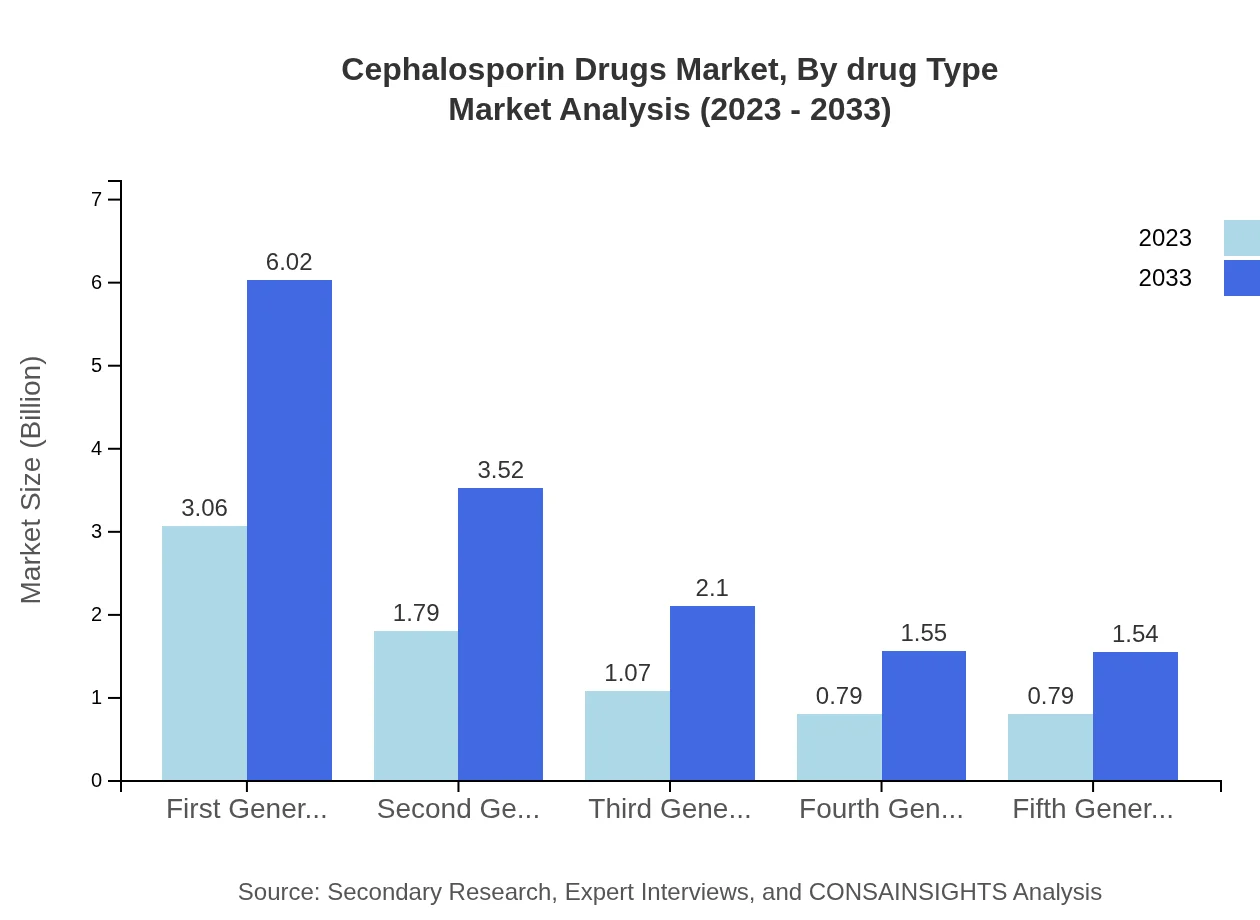
Cephalosporin Drugs Market Analysis By Drug Type
The market is segmented by drug type, including first to fifth generations. The first-generation cephalosporins maintain a significant market share, accounting for 40.84% of the market in 2023, valued at $3.06 billion and expected to grow to $6.02 billion by 2033. This is followed by second and third generations, demonstrating a shift in preference towards effective therapeutic options.
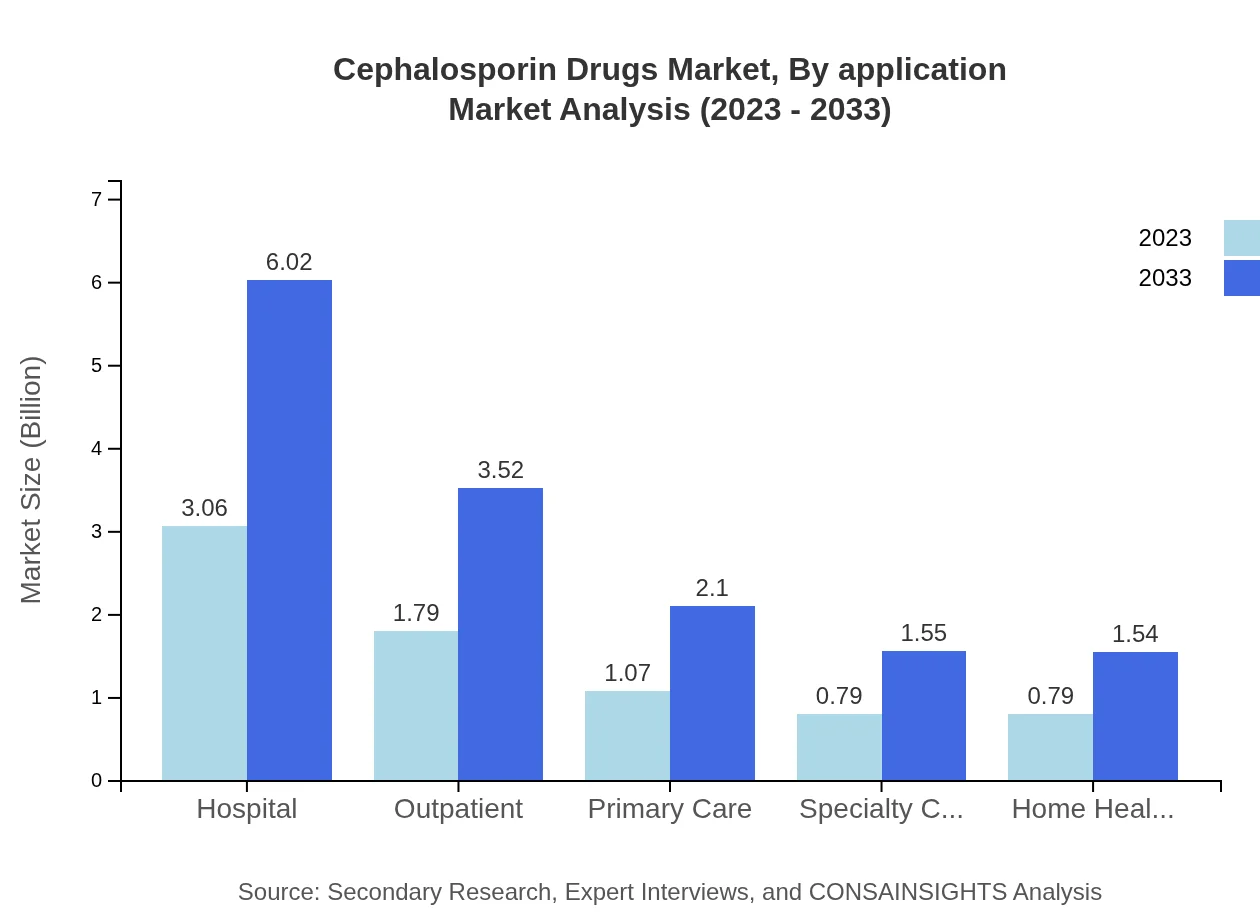
Cephalosporin Drugs Market Analysis By Application
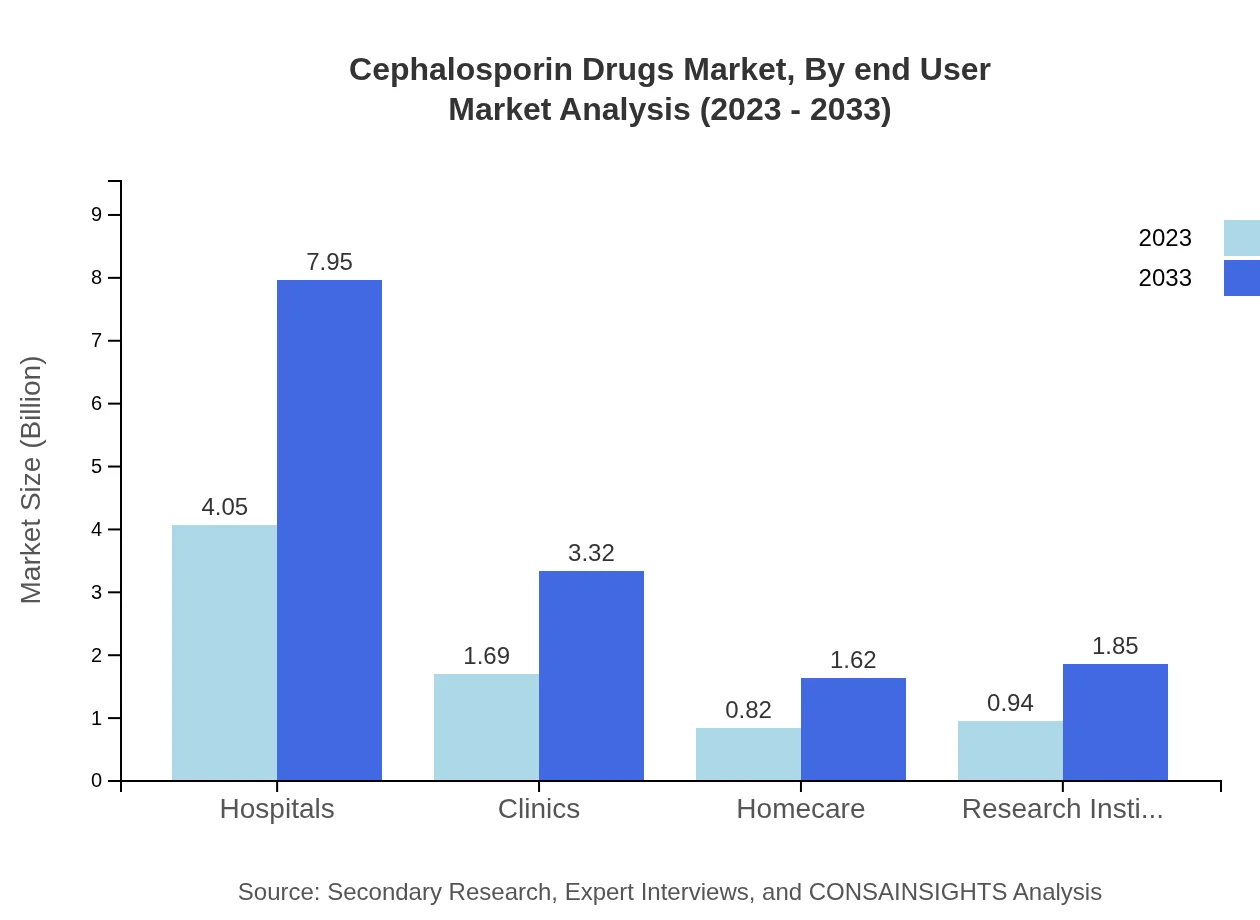
In terms of application, hospitals represent the largest segment, constituting 53.95% of the market share in 2023, valued at $4.05 billion and projected to rise to $7.95 billion by 2033. Clinics and homecare are also significant, projected to increase demand as healthcare evolves towards community settings.
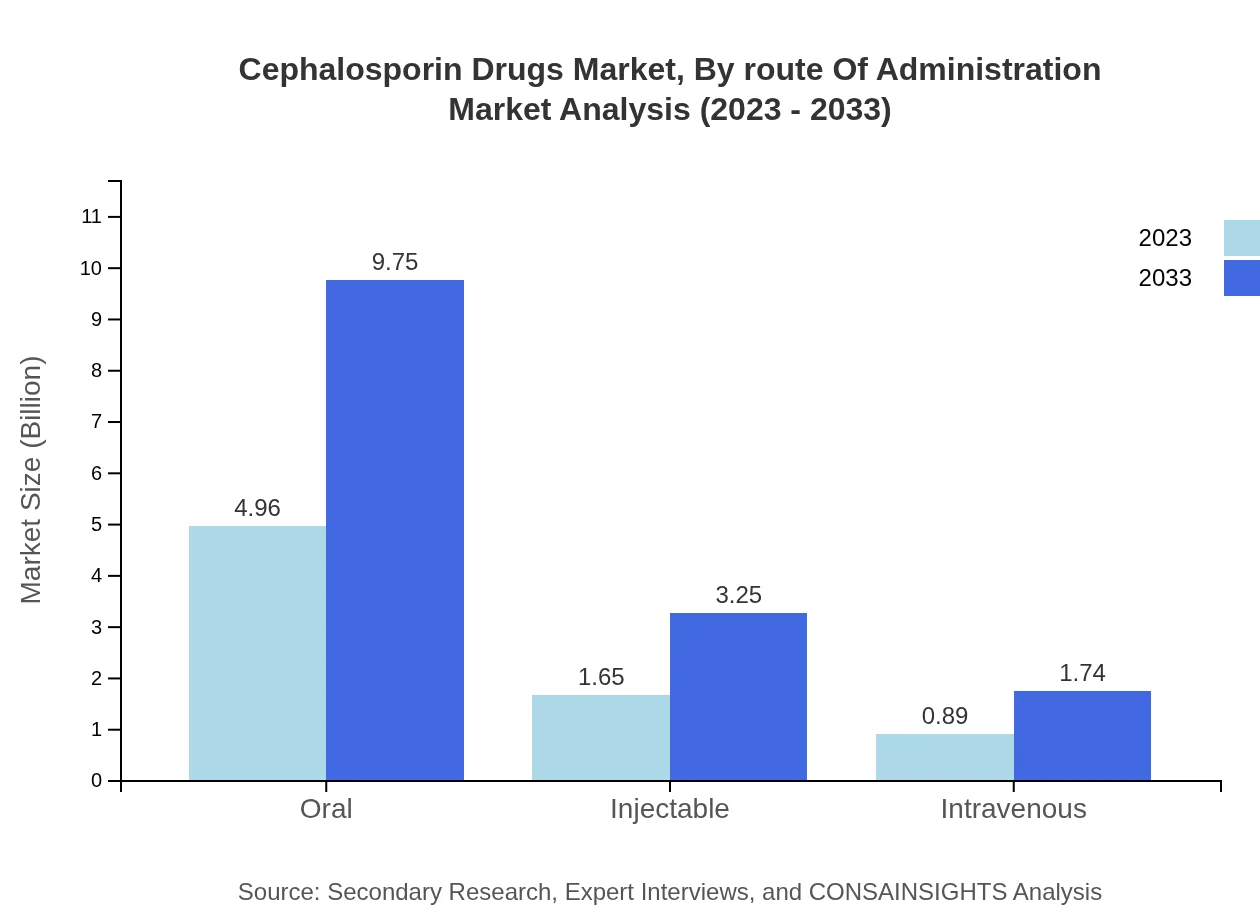
Cephalosporin Drugs Market Analysis By Route Of Administration
The route of administration segment shows oral forms dominating the market share, accounting for 66.14% in 2023. Injectable forms follow closely, represented 22.05%. The focus on non-invasive treatment options and advancements in oral formulations continues to drive this trend.
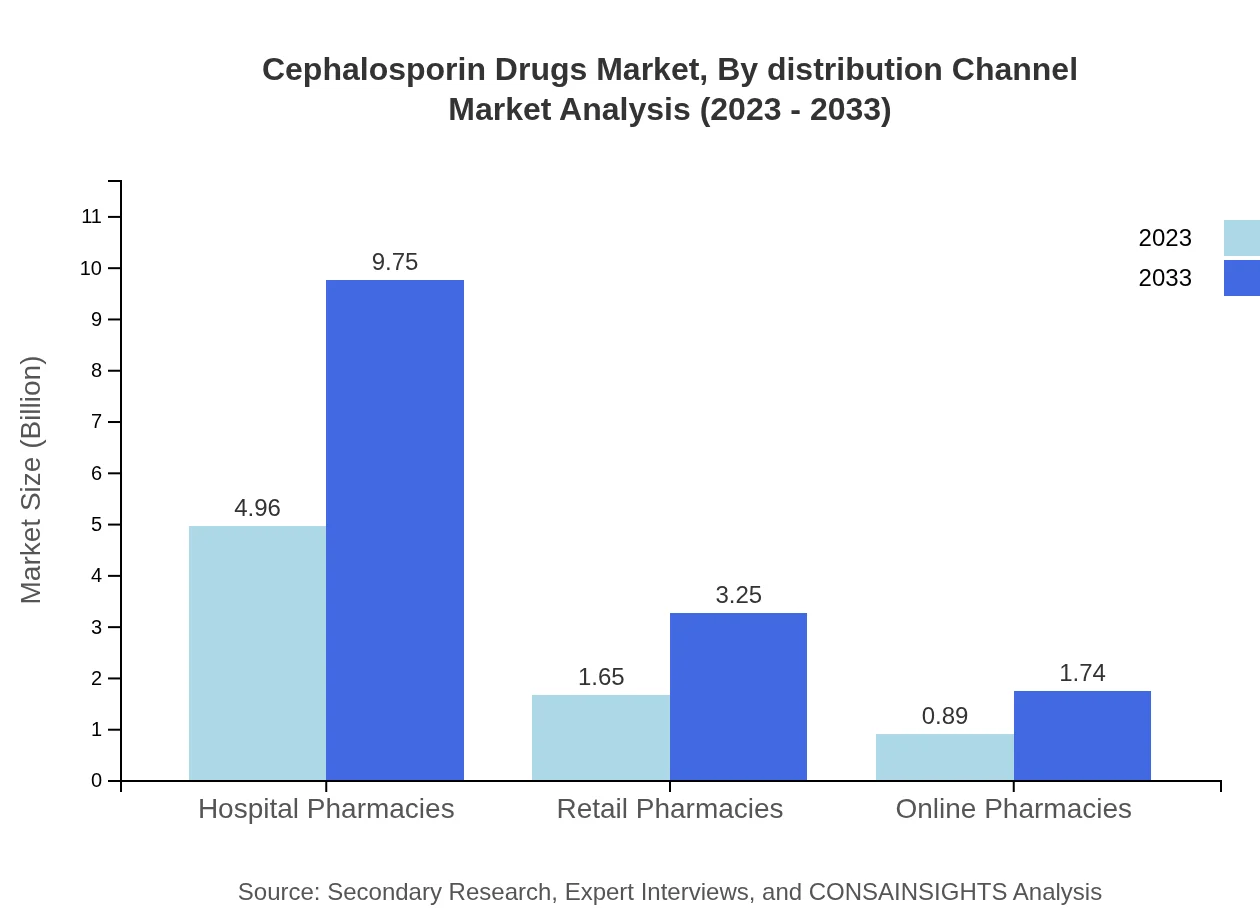
Cephalosporin Drugs Market Analysis By Distribution Channel
Distribution channels include retail and online pharmacies, with hospital pharmacies dominating at a 66.14% market share in 2023. The trend towards online sales channels is expected to grow significantly, driven by consumer preferences for convenience.
Cephalosporin Drugs Market Analysis By End User
End-users of these drugs include hospitals, clinics, and research institutions. Hospital pharmacies represent the largest segment, providing access to essential medications amid rising healthcare demands.
Cephalosporin Drugs Market Trends and Future Forecast
Tell us your focus area and get a customized research report.
Global Market Leaders and Top Companies in Cephalosporin Drugs Industry
Pfizer Inc.:
A leading global biopharmaceutical company, Pfizer plays a significant role in the development and supply of innovative cephalosporin drugs, addressing various bacterial infections.Merck & Co.:
Merck is a prominent player in the cephalosporin market, committed to advancing antibiotic treatments and improving formulations for better patient outcomes.Novartis AG:
Novartis has significantly invested in antibiotic research, focusing on cephalosporins to meet the rising global demand for effective treatment options.Johnson & Johnson:
Johnson & Johnson's pharmaceutical division is dedicated to creating new therapies, including cephalosporin antibiotics, enhancing global health standards.Roche Holdings AG:
Roche is involved in innovative antibiotic development, including various cephalosporin drugs aimed at treating resistant bacterial strains.We're grateful to work with incredible clients.









FAQs
What is the market size of cephalosporin drugs?
The cephalosporin drugs market size is estimated at $7.5 billion in 2023, with a projected growth at a CAGR of 6.8% over the next decade, indicating strong demand and expansion across various healthcare sectors.
What are the key market players or companies in the cephalosporin drugs industry?
The cephalosporin drugs industry is dominated by major pharmaceutical companies such as Pfizer, Merck & Co., GSK, and Johnson & Johnson, which have extensive portfolios and R&D investments in antibiotic solutions.
What are the primary factors driving the growth in the cephalosporin drugs industry?
Key growth drivers include the increasing prevalence of bacterial infections, advancements in pharmaceuticals leading to new drug formulations, and rising healthcare expenditures that enhance access to antibiotics.
Which region is the fastest Growing in the cephalosporin drugs market?
The Asia Pacific region is the fastest-growing market, expected to expand from $1.49 billion in 2023 to $2.93 billion by 2033, fueled by improving healthcare infrastructure and rising demand for antibiotics.
Does ConsaInsights provide customized market report data for the cephalosporin drugs industry?
Yes, ConsaInsights offers customized market report data tailored to client specifications, addressing specific inquiries and providing detailed insights into industry trends, competitors, and market dynamics.
What deliverables can I expect from this cephalosporin drugs market research project?
Expect comprehensive deliverables, including market size data, growth forecasts, competitive analysis, and segmented insights, focusing on various regions, demographics, and delivery methods for cephalosporin drugs.
What are the market trends of cephalosporin drugs?
Market trends indicate a shift towards increased usage of broad-spectrum antibiotics, growth in outpatient care settings, and greater investment in antibiotic stewardship programs to combat drug resistance.